Abstract
Studies by the International Working Group showed that the prognosis of myelofibrosis patients is predicted by the Dynamic International Prognostic Scoring System (DIPSS) risk categorization, which includes patient age, constitutional symptoms, hemoglobin, leukocyte count, and circulating blasts. We evaluated the prognostic usefulness of the DIPSS in 170 patients with myelofibrosis, 12 to 78 years of age (median, 51.5 years of age), who received hematopoietic cell transplantation (HCT) between 1990 and 2009 from related (n = 86) or unrelated donors (n = 84). By DIPSS, 21 patients had low-risk disease, 48 had intermediate-1, 50 had intermediate-2, and 51 had high-risk disease. Five-year incidence of relapse, relapse-free survival, overall survival, and nonrelapse mortality for all patients were 10%, 57%, 57%, and 34%, respectively. Among patients with DIPSS high-risk disease, the hazard ratio for post-HCT mortality was 4.11 (95% CI, 1.44-11.78; P = .008), and for nonrelapse mortality was 3.41 (95% CI, 1.15-10.09; P = .03) compared with low-risk patients. After a median follow-up of 5.9 years, the median survivals have not been reached for DIPSS risk groups low and intermediate-1, and were 7 and 2.5 years for intermediate-2 and high-risk patients, respectively. Thus, HCT was curative for a large proportion of patients with myelofibrosis, and post-HCT success was dependent on pre-HCT DIPSS classification.
Introduction
Myelofibrosis is a myeloproliferative disorder/neoplasm characterized by the presence of megakaryocyte proliferation and nuclear atypia with reticulin and collagen fibrosis.1 Hematopoietic cell transplantation (HCT) offers potentially curative therapy but may carry a substantial risk of nonrelapse mortality (NRM).2-5 The development of new nontransplantation therapies for myelofibrosis, such as immunomodulatory derivatives6-10 and JAK2 inhibitors,11,12 although not curative, may improve quality of life and prolong survival with only limited toxicity. A pivotal issue in the treatment of patients with myelofibrosis, therefore, is the optimal timing of HCT. This is an important question because once patients have progressed to acute myeloid leukemia (AML), the probability of a successful HCT declines significantly13 compared with patients transplanted before transformation to AML.3 Several prognostic scoring systems have been described that provide insight into the natural history of myelofibrosis and may ultimately be useful in determining the optimal timing of HCT.
The Dupriez classification considered hemoglobin and total white blood cell count as prognostic factors14 and divided patients into 3 risk groups. The team at the Mayo Clinic suggested that adding monocyte and platelet counts to hemoglobin and white blood cell counts resulted in a superior prognostic scoring system with improved separation of distinct risk groups.15 The International Working Group has shown more recently that age more than 65 years, hemoglobin less than 10 g/dL, white blood cell count more than 25 × 109/L, circulating blasts more than or equal to 1%, and presence of constitutional symptoms are relevant prognostic factors in patients with myelofibrosis.16 Based on these variables, the International Prognostic Scoring System (IPSS) generated 4 risk categories with projected median survivals of 125, 95, 48, and 27 months, respectively. A time-dependent risk evaluation proved the dynamic nature of the IPSS, which is now referred to as dynamic IPSS (DIPSS).17 The authors showed that the variables included in the IPSS maintained their relevance over time and that progression to higher-risk categories was associated with a higher risk of mortality. At the time of reporting, the anticipated median survival estimates for the 4 DIPSS risk categories were not yet reached in the low-risk group, and were 14.2 years, 4 years, and 1.5 years, for the intermediate-1, intermediate-2, and high-risk groups, respectively. The dynamic nature of the DIPSS suggests that it may be useful for making treatment decisions. Additional analyses have shown that cytogenetics, transfusion status, and platelet counts add prognostic information to the DIPSS (DIPSS Plus).18 There are limited data regarding the utility of the IPSS and DIPSS when advising patients with myelofibrosis regarding HCT. The IPSS proposal included only 5 patients who received an allogeneic HCT, and the DIPSS cohort censored the 8 patients who underwent allogeneic HCT. Given these data, we sought to validate the DIPSS in a population of patients who underwent HCT as treatment for myelofibrosis. In addition, because it has become increasingly clear that patient-specific factors may be as important as disease-specific parameters in predicting transplantation outcome,19 we sought to assess the impact of the hematopoietic cell transplantation-specific comorbidity index (HCT-CI) in parallel to the DIPSS on HCT outcomes and determine possible interactions between the parameters included in the 2 instruments.
Methods
Data collection
We retrospectively surveyed patients who received an allogeneic or syngeneic HCT at the Fred Hutchinson Cancer Research Center (FHCRC) for a diagnosis of myelofibrosis from March 1990 through November 2009. The diagnosis of myelofibrosis was based on the revised World Health Organization (WHO) 2008 classification,1 which was applied retrospectively to patients transplanted before 2008. Patients with both primary myelofibrosis and secondary myelofibrosis from a preceding diagnosis of essential thrombocythemia or polycythemia vera were included in this analysis. Time from diagnosis to HCT was based on the first marrow biopsy that confirmed the diagnosis of myelofibrosis. Patients whose disease had ever progressed to leukemia (≥ 20% marrow or blood myeloblasts) were excluded. The degree of fibrosis was based on a bone marrow biopsy obtained within 25 days before HCT.20 The DIPSS scores were assessed in all patients based on parameters at the time of HCT. Assessment included a retrospective chart review of all clinic notes before HCT. The presence or absence of constitutional symptoms was based on standardized review of systems forms taken on all patients, which included questions regarding the presence of weight loss, excessive sweats, and unexplained fever. Cytogenetic risk was based on DIPSS Plus risk categories.21 JAK2-V617F mutational status was based on both outside and internal laboratory review. HCT-CI scores were determined before HCT as previously described.22 Marrow examination, including morphology, flow cytometry, and cytogenetic analyses, was performed on all surviving patients at 1, 3, 6, and 12 months after HCT. Cytogenetic analysis was performed by standard banding technique; in addition, patients who had a known cytogenetic marker, for which probes were available, had FISH performed. FISH testing was not routinely performed in patients with normal cytogenetics. Donor/patient chimerism analyses on unfractionated marrow cells and sorted peripheral blood cells (CD3+; CD33+) were performed 1, 3, 6, and 12 months after HCT in all surviving patients who received reduced-intensity conditioning.23
Patients or legal guardians for patients younger than 18 years gave informed consent to use medical information for the purposes of research approved by the Institutional Review Board of the FHCRC in accordance with the Declaration of Helsinki.
Patient and disease characteristics
A total of 170 patients underwent allogeneic (n = 167) or syngeneic (n = 3) HCT for a diagnosis of myelofibrosis at the FHCRC between March 1990 and November 2009 (Table 1). The median time from diagnosis was 15 months. As expected in a population of patients who undergo HCT, only 18% of the 114 patients who could be scored for HCT-CI had scores more than or equal to 4. Seventy patients had secondary myelofibrosis, primarily resulting from a prior diagnosis of polycythemia vera or essential thrombocythemia. Just more than half of the patients had normal cytogenetics. There were 3 patients in whom no metaphase spreads for cytogenetic studies could be obtained. A reliable spleen examination could not be performed in 3 patients because of body habitus. The most common DIPSS risk factor was hemoglobin less than 10 g/dL. Compared with nontransplantation series,16 the distribution of patients in the present series was shifted toward higher-risk categories and younger age.
Transplant characteristics
Most patients received G-CSF–mobilized peripheral blood stem cells (PBSCs) after conditioning with high-dose busulfan (BU)–containing regimens (Table 2). The majority of patients who underwent allogeneic HCT were conditioned with oral BU (prescribed dose, 16 mg/kg) with dose adjustments targeting a steady-state plasma concentration of 800 to 1000 ng/mL. In patients who received targeted BU, the median BU concentration at steady state was 852 ng/mL (range, 690-1029 ng/mL). The 3 syngeneic transplant recipients received oral BU at 16 mg/kg (n = 2) or oral BU combined with 12 Gy total body irradiation (TBI; n = 1). Intensity of conditioning was based on active protocols at time of HCT and on the patients underlying comorbidities and age. Stem cells were infused within 24 hours of completion of TBI or within 36 to 48 hours after the last dose of chemotherapy. All recipients received T cell–replete grafts. ABO incompatibility between donor and patient was managed as previously described.24
HLA typing was performed as previously described.25 The median marrow cell dose was 7.8 × 108 (range, 1.1-23.5 total nuclear cells/kg), and the median PBSC dose was 8.4 × 106 (range, 0.57-27.04 CD34+ cells/kg). The target cell dose for both related and unrelated PBSC transplants was 5 × 106 CD34+ cells/kg, and for marrow stem cells 4 × 108 total nuclear cells/kg. Cyclosporine combined with methotrexate was the most frequently used GVHD prophylaxis.26
Supportive care
All patients had central venous access lines placed before HCT. Infection prophylaxis was given according to standard institutional practices, including broad-spectrum antibiotics during the neutropenic period, fluconazole, or other azole compounds for fungal prophylaxis, trimethoprim-sulfamethoxazole for prevention of Pneumocystis jiroveci pneumonia, and acyclovir for viral prophylaxis. Surveillance for CMV reactivation or acquisition was carried out weekly by shell vial-based or PCR testing of plasma samples until day 100, and preemptive anti-CMV therapy was initiated in patients who showed evidence of CMV reactivation.27 Neither G-CSF nor GM-CSF was routinely administered after stem cell infusion.
Definition of end points
The primary endpoint in this analysis was overall survival (OS). Additional endpoints included time to engraftment, incidence of acute and chronic GVHD, NRM, and relapse-free survival (RFS). Among patients who received high-dose conditioning with BU, TBI, or treosulfan containing regimens, time to neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count of 0.5 × 109/L or greater. Among patients who were conditioned with reduced-intensity regimens, primary graft failure was defined as less than 5% CD3+ donor T cells on day 28, and graft rejection (or secondary graft failure) was defined as less than 5% CD 3+ donor T cells at any time after initial engraftment.28 Chimerism studies were routinely performed in patients who received reduced-intensity conditioning, but not in patients who were conditioned with higher intensity regimens. Overall survival (OS) was the time from HCT until death or date of last contact. Relapse-free survival (RFS) was the time from HCT until death, relapse, or date of last contact. All patients were scheduled for marrow evaluations with aspiration and biopsy at 1, 3, and 12 months after HCT for morphologic, flow cytometric, and cytogenetic analyses. Reappearance or worsening of fibrosis after initial clearance or improvement of marrow fibrosis or the detection of pre-HCT abnormalities by flow cytometry or cytogenetic analysis was considered evidence of relapse.3 In the absence of other evidence of disease recurrence, persistence of fibrosis alone was not considered sufficient evidence of relapse or progression because fibrosis may resolve slowly over variable time intervals after HCT.29 Acute GVHD and chronic GVHD were diagnosed, graded, and treated as previously described.30,31 In patients who died after relapse, relapse was considered the cause of death regardless of the proximate cause of death. Similarly, among patients who died with graft failure or graft rejection, graft failure/rejection was considered the cause of death regardless of the proximate cause of death. Patients who were receiving immunosuppressive therapy for GVHD and died from infection were considered to have died from GVHD. Infection was considered the cause of death only in the absence of relapse, graft failure/graft rejection, and GVHD requiring immunosuppressive therapy. Multiorgan failures, including veno-occlusive disease (sinusoidal obstruction syndrome) of the liver and diffuse alveolar hemorrhage, were listed as the cause of death if they occurred in the absence of active infection or relapse.32
Statistical analysis
Estimates of OS and RFS were calculated using the method of Kaplan and Meier. Relapse or death, whichever occurred first, was considered as failure for the endpoint of RFS. Cumulative incidence curves were used to estimate the probabilities of relapse and NRM.33 Death without relapse was treated as a competing risk for relapse, and relapse was considered a competing risk for NRM. Adjusted survival estimates were calculated using the method of Storer et al.34 The statistical significance of differences in event rates between DIPSS categories was evaluated with the Cox regression model. Several explanatory variables were examined for association with outcome after adjusting for DIPSS; these variables included patient age, donor type, use of high-dose conditioning, HCT-CI,35 and prior splenectomy. All reported 2-sided P values from regression models were derived from the Wald test. A P value of less than .05 was considered significant. The statistical analysis was performed on SAS Version 9 software (SAS Institute). Results were analyzed as of July 2011.
Results
Engraftment
One syngeneic recipient conditioned with high-dose TBI/BU failed to achieve engraftment. Among 167 allogeneic recipients, 4 had primary graft failure and 8 had graft rejection/secondary graft failure; 2 patients died before day 28 without hematopoietic recovery or GVHD. Among the 8 patients with graft rejection, the median time to graft rejection was 53 days (range, 29-178 days). Of the 12 allogeneic recipients with graft failure, 4 had received low-dose TBI conditioning (of a total of 13), and 8 had been conditioned with high-dose BU (n = 7) or high-dose TBI (n = 1; of 152). There was no correlation between degree of fibrosis on pre-HCT bone marrow morphology and time to engraftment among the 153 allogeneic recipients with sustained engraftment (P = .83). There was no association between degree of fibrosis or BU steady state and graft rejection/failure. Among patients who underwent splenectomy before HCT, engraftment occurred at a median of 19 days (range, 12-29 days). Among patients who were not splenectomized, engraftment occurred at a median of 18 days (range, 10-51 days). Bone marrow stem cell recipients engrafted at a median of 20 days (range, 10-51 days). PBSC recipients engrafted at a median of 19 days (range, 10-35 days).
GVHD
Among the 160 allogeneic transplant recipients who were at risk and were graded for acute GVHD, the overall incidence of grades 2 to 4 (3 or 4) acute GVHD was 68% (18%). The corresponding incidences were 63% (11%) with HLA-identical sibling donors, 73% (21%) with unrelated donors, and 57% (43%) with HLA-mismatched related donors. Chronic GVHD was diagnosed in 97 of 153 patients at risk (63%) and was limited in 13 (8%) and extensive in 84 patients (55%). There was no correlation between DIPSS score and the incidence or severity of acute or chronic GVHD.
Relapse, RFS, OS, and NRM
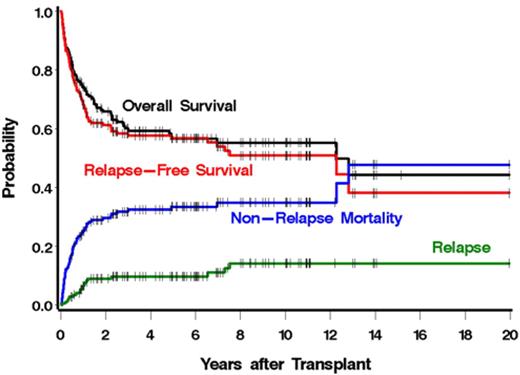
Estimates of relapse, RFS, OS, and NRM are summarized in Figure 1. With a median follow-up of 5.9 years (range, 0.5-20.0 years), the 1-year cumulative incidences of these endpoints were 7%, 68%, 74%, and 26%, respectively. Five-year estimates were 10%, 57%, 57%, and 34%, respectively. There were 19 patients who relapsed. Overall, 72 patients died, including 13 with relapse. The causes of death are summarized in Table 3. There was a nonsignificant trend toward increased mortality among patients with secondary myelofibrosis compared with patients with primary myelofibrosis (hazard ratio [HR] = 1.59, 95% CI, 0.97-2.62, P = .07). There were no significant differences in relapse, RFS, or NRM between patients with primary myelofibrosis versus secondary myelofibrosis.
OS, RFS, relapse, and NRM among 170 patients with myelofibrosis after allogeneic HCT.
OS, RFS, relapse, and NRM among 170 patients with myelofibrosis after allogeneic HCT.
We also examined the impact of conditioning regimens on survival. After adjustment for DIPSS, a regimen of cyclophosphamide, 120 mg/kg followed by BU, 16 mg/kg intravenously (targeted to 800-1000 ng/mL), resulted in the lowest mortality (HR = 0.57; 95% CI, 0.18-1.77; P = .32). The highest mortality rate was observed with a regimen that combined oral BU, 7 mg/kg, with high-dose (12 Gy) TBI (HR = 1.30; 95% CI, 0.47-3.60; P = .62). Outcome with a reduced-intensity conditioning regimen combining 90 mg/m2 fludarabine and 2 to 3 Gy TBI served as the referent group for both comparisons. No significant differences in mortality between any of the conditioning regimens were noted; however, small numbers of patients in some regimens prevented a formal statistical comparison.
DIPSS
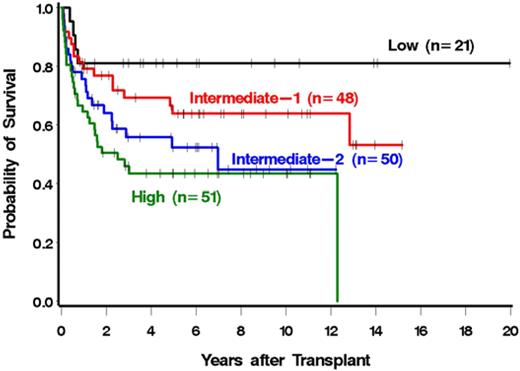
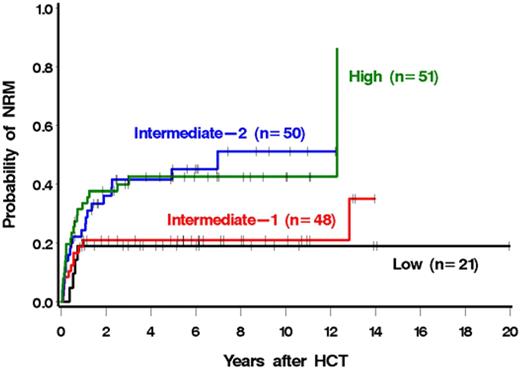
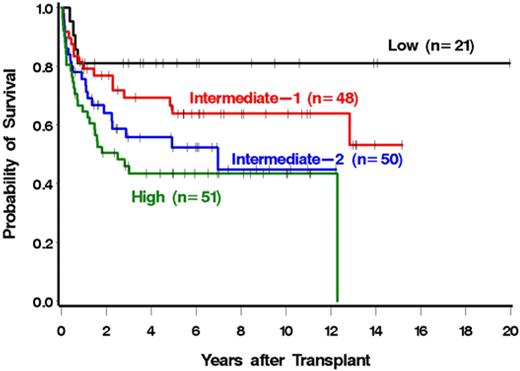
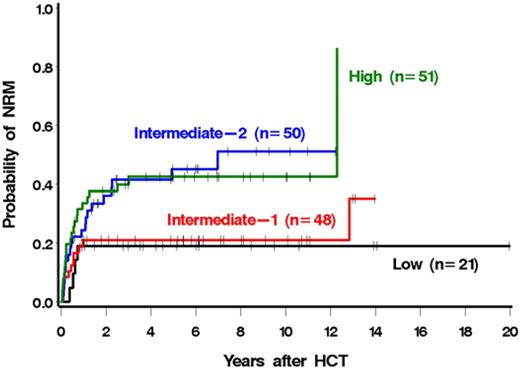
The risk of mortality was correlated with the DIPSS risk category (Figure 2; Table 4). In particular, patients with high-risk disease were 4.11 times more likely to die after HCT than patients with low-risk disease (Table 4). Similar results were seen for NRM (Figure 3; Table 4). Patients with high-risk disease were 3.41 times more likely to die from nonrelapse causes than patients with low-risk disease. The association with relapse was not as striking, although relapse was less frequent with lower-risk disease (Table 4). Among low and intermediate-1 risk patients, median survival had not been reached at a median follow-up of 5.2 years (range, 1-20 years) and 6.3 years (range, 0.8-15.2 years), respectively. The median survivals among intermediate-2 and high-risk patients were 7 years and 2.5 years with median follow-ups of 4.9 years (range, 0.5-12.2 years) and 5.7 years (range, 0.6-11.1 years), respectively. The single late death (12 years after HCT) of a patient in the high-risk group was the result of infectious complications after surgery and was considered unrelated to prior HCT.
We next examined a possible association of each individual DIPSS component with outcome (Table 5). Four of the 5 components were associated with increased mortality among patients possessing the high-risk value of the parameter relative to those possessing the low-risk value. Although not every DIPSS factor reached statistical significance, statistical power was relatively limited for some (eg, there were only 9 patients in the high-risk age category). The only high-risk DIPSS component that was not associated with higher mortality relative to its low-risk category was a high leukocyte count. Similar associations were seen for relapse plus death and NRM and, to a lesser degree, for relapse.
Association of non-DIPSS factors with transplantation outcomes
After adjustment for the DIPSS, we examined donor type, conditioning intensity, HCT-CI, stem cell source, JAK2-V617F mutational status, and splenectomy as additional factors that might affect outcome of HCT (Table 6). No factor showed a significant association. After adjustment for DIPPS, patients who had undergone splenectomy before HCT were at half the risk of mortality compared with patients who did not have a splenectomy. When adjusting by both DIPSS and HCT-CI, we found that patients who had a splenectomy were at significantly lower risk for mortality compared with patients who have not had a splenectomy (HR = 0.44, 95% CI, 0.2-0.95, P = .04).
HCT-CI scores were available for 114 patients (63%). Increasing HCT-CI scores (modeled as a continuous linear variable) were associated with increased overall mortality (P = .04) and NRM (P = .02) in unadjusted models. However, after adjustment for DIPSS, the significance of these associations was diminished (P = .13 and P = .08, respectively). Similarly, the associations of DIPSS categories with transplantation outcomes were less significant after adjustment for HCT-CI, at least in part the result of a significant positive correlation between DIPSS and HCT-CI (r = 0.22, P = .02). For example, among patients with an HCT-CI score in the high-risk DIPSS category the likelihood of death was 3.13 times greater than the low-risk group after adjustment for HCT-CI, compared with a hazard ratio of 3.49 without adjustment for HCT-CI. Similarly, among the patients with HCT-CI scores, NRM among those with high-risk disease by DIPSS was 2.82 times that of low-risk patients; however, after adjustment for HCT-CI, the hazard ratio was reduced to 2.46. Thus, both the DIPSS and HCT-CI were associated with overall mortality and NRM when considered separately from each other, but because of the correlation between them, it is difficult to separate out the effects of each. Among all components of the DIPSS, constitutional symptoms were most closely associated with a higher HCT-CI (mean HCT-CI 2.09 vs 1.31, P = .02). Older patients had, on average, a higher HCT-CI, but the association was not statistically significant (mean HCT-CI in patients > 65 years 2.75 vs 1.66 in patients < 65 years, P = .09). There was a positive correlation between degree of fibrosis and DIPSS, but this correlation did not reach statistical significance (r = 0.14, P = .14).
Effect of transplantation year
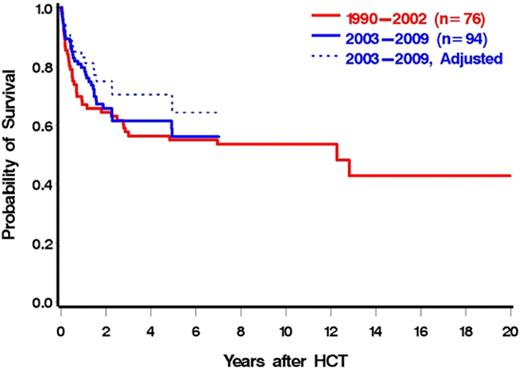
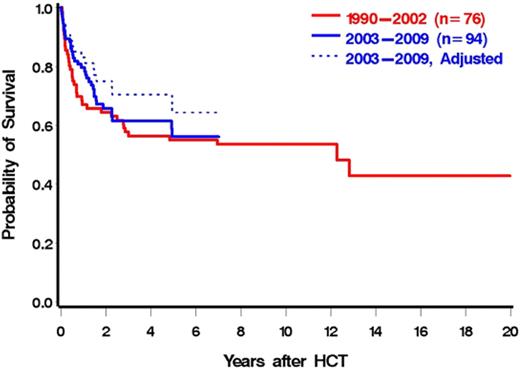
We speculated that advancements in HCT, including improved antimicrobial therapy, improved GVHD prophylaxis, and decreased toxicity with conditioning, would lead to lower NRM resulting in better OS in more recently transplanted patients. Unexpectedly, the unadjusted overall mortality and NRM were largely unaffected by year of transplantation (P = .95 for overall mortality, P = .75 for NRM when year was modeled as a continuous linear variable). However, after adjustments for DIPSS, HCT-CI, and donor type, these associations, although not reaching statistical significance, were considerably stronger (P = .24 for overall mortality, P = .14 for NRM). Figure 4 illustrates OS by year of HCT (2003-2009 vs 1990-2002). Although the unadjusted rate of overall mortality was similar for the 2 time intervals (HR = 0.88; 95% CI, 0.55-1.41; P = .59), after adjustments for DIPSS, HCT-CI, and donor status, the difference between the groups was increased (HR = 0.69; 95% CI, 0.38-1.29; P = .25). This observation was in part the result of a correlation between increasing HCT-CI and more recent years of HCT, with both modeled as continuous variables (r = 0.23, P = .01). These data support the concept that in recent years patients in progressively higher-risk categories as defined by HCT-CI have been referred for HCT.
OS by year of HCT (1990-2202 vs 2003-2009). Dotted line is adjusted for HCT-CI, DIPSS, and donor status.
OS by year of HCT (1990-2202 vs 2003-2009). Dotted line is adjusted for HCT-CI, DIPSS, and donor status.
Discussion
The advantages of an accurate prognostic model include the ability to estimate the patient's life expectancy and to weigh the potential benefits of available therapy. In general, the more aggressive a disease, the more likely physicians and patients are to consider high-risk therapeutic interventions. HCT may be curative for patients with myelofibrosis, but the risks include treatment-related toxicity and the development of acute and chronic GVHD, which can contribute to considerable morbidity and mortality. Using an analysis of data on patients with myelofibrosis reported to the Center for International Blood and Marrow Transplant Research, Ballen et al showed a 1-year NRM of 27% for HLA-identical HCT and 43% for HLA-matched unrelated HCT,5 underscoring the fact that HCT can be associated with potentially fatal complications. Progress in HCT over the past 2 decades has led to markedly improved HCT outcomes.36,37 Advances include the development of novel HCT preparative regimens, improved antimicrobial prophylaxis, and improved GVHD prophylaxis, which have led to 1-year NRM of less than 20% and even less than 10%.4 Concurrently, nontransplantation therapies, such as immunomodulatory derivatives and JAK2 inhibitors, have been developed which, although not curative, have resulted in improvement of hematologic parameters and patient symptomatology with limited toxicity.9-12 These findings lead to difficult patient management decisions. Non-HCT modalities may improve symptoms and stabilize the disease for variable periods of time; however, with this approach, patients are probably offered HCT, the only curative therapy for myelofibrosis, at a more advanced stage of their disease, when outcome is expected to be inferior to what could have been achieved with an earlier HCT.38 As we expect additional new treatment options to be developed, it will be increasingly important to integrate parameters of prognostic relevance into both the HCT and non-HCT settings. Therefore, we sought to determine the impact of the DIPSS, widely used to counsel patients with myelofibrosis, on post-HCT outcomes.
The present results show that the DIPSS classification was associated with HCT outcomes, such as overall mortality, failure because of relapse or death, and NRM. Of note, the global association between DIPSS and relapse was not statistically significant, although the difference between the low-risk and high-risk groups did yield a P value of .05, and patients in higher-risk DIPSS categories experienced more relapses than patients in the lower-risk categories. The median life expectancies according to DIPSS categories for patients with myelofibrosis treated without HCT were “not reached,” 14.2 years, 4 years, and 1.5 years for low, intermediate-1, intermediate-2, and high-risk, respectively, whereas they were “not reached,” “not reached,” 7 years, and 2.5 years after HCT. Direct comparison of these median life expectancies are not advisable as there may have been additional factors beyond the DIPSS that lead to a selection bias among patients offered HCT.
We examined additional factors, including donor type, HCT-CI, stem cell source, JAK2-V617F mutational status, and splenectomy, to determine whether there was any association with post-HCT outcomes. Alchalby et al previously showed that patients with wild-type JAK2-V617F had an inferior survival after allogeneic HCT.39 Similar to other investigators,40 we were not able to confirm these findings; however, this result could be an effect of small sample size. Pre-HCT splenectomy was the only variable significantly associated with mortality after adjustment for DIPSS risk. After adjusting for disease severity, patients who had undergone splenectomy before HCT had a lower post-HCT mortality than patients with their spleens intact. This finding was in contrast to our earlier report in smaller numbers of patients where we had failed to show an advantage or disadvantage of pre-HCT splenectomy.41 Splenectomy may have reduced peritransplantation morbidity and accelerated post-HCT hematopoietic recovery, although in our previous analysis the differences in engraftment kinetics of platelets and neutrophils were minimal.3 Alternatively, there may have been a selection bias with only relatively healthy patients being referred for splenectomy. The significantly lower mortality associated with splenectomy despite adjustment for DIPSS and HCT-CI argues against such a bias. In this analysis, patients who underwent splenectomy must have survived that intervention to proceed to HCT. We do not have access to data on patients who underwent splenectomy and were not referred for HCT, possibly because of complications related to the procedure. Increased HCT-CI scores were associated with increased overall mortality and NRM. The inclusion of HCT-CI with the DIPSS decreased the significance of DIPSS score because of the correlation between DIPSS and HCT-CI. Combining external markers of overall health as measured by the HCT-CI and disease specific markers as reflected in the DIPSS may allow the determination of the best possible therapy for each individual patient.
Our data indicate that more recently transplanted patients presented for HCT with more advanced disease and generally had more comorbidities than patients transplanted in earlier years: patients in the more recent HCT cohort (1990-2002 vs 2003-2009) had nonsignificantly higher HCT-CI and DIPSS scores. These factors tend to obscure progress that may have been made. Indeed, the adjusted data, although not statistically significant, showed a progressive decrease in NRM and increase in survival over the past decade. These findings are similar to those shown recently in large cohorts of patients with various malignancies.36,37 In the future, as new therapies become available, it is probable that patients will be referred for HCT later in the course of their disease.
In conclusion, offering patients with myelofibrosis HCT earlier in their disease course resulted in superior outcomes. However, patients with early-stage disease probably have a longer life expectancy (than patients with advanced disease) without HCT. Based on these data, we recommend that patients with intermediate-2 or high-risk disease by DIPSS be considered for HCT. Patients with lower scores should be advised individually. However, all patients with low-risk and intermediate-1 risk disease who receive alternative treatments should be followed closely for evidence of disease progression. Future studies should use the DIPSS to assess the optimal timing of interventions, such as HCT. Additional work is needed to provide more specific recommendations to patients and their physicians regarding HCT. The present study represents a contribution to those efforts by confirming the validity of the DIPSS in patients with myelofibrosis who undergo HCT.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the transplant teams at the FHCRC and the Seattle Veterans Administration Puget Sound Health Care System for their contributions, all patients for participation in clinical trials, Wendy Wilson and Carlos Breton for maintaining the database, and Bonnie Larson and Helen Crawford for their help with manuscript preparation.
This work was supported in part by the National Institutes of Health (grants HL084054, HL36444, CA18029, CA15704, and HL088021).
National Institutes of Health
Authorship
Contribution: B.L.S. designed the study, collected and interpreted the data, and wrote the paper; T.A.G. was the principle statistician for the analysis and analyzed the data; M.L.S. provided the HCT-CI scores; A.R.R., D.M., and T.R.C. assisted in data collection; M.L.L. assisted in data interpretation and writing the paper; J.G. and R.S. assisted in writing the paper; B.M.S., J.P.R., and F.R.A. assisted in data analysis and writing the paper; V.B.-A. performed the JAK 2 v617f mutational analysis on patients; and H.J.D. assisted in study design, data collection, data interpretation, and writing the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bart L. Scott, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D1-100, Seattle, WA 98109-1024; e-mail: bscott@fhcrc.org.