Abstract
The genetic basis underlying inferior outcome of adolescent and young adult acute lymphoblastic leukemia (AYA ALL) as compared to childhood cases is largely unknown. To comprehensively characterize the genetic landscape of AYA ALL we studied 423 adolescent (16-21 yrs; median 17.7±1.3 yrs) and 250 young adult (21-39 yrs; median 28.3±7.0 yrs) samples from the Children's Oncology Group high-risk trial AALL0232, St Jude Children's Research Hospital Total XV and XVI, Eastern Cooperative Oncology Group E2993, MD Anderson Cancer Center and the Alliance - CALGB trials. Single nucleotide polymorphism (SNP) microarray analysis and gene expression profiling were performed to identify copy number alterations and distinct genetic subgroups. Samples were also sub classified using hierarchical clustering, ROSE outlier and PAM analysis of gene expression profiling data. Sequence mutation analysis was performed on candidate genes known to be mutated in pediatric ALL (including IKZF1, PAX5, JAK1/2, NRAS, KRAS, FLT3, IL7R, SH2B3, TP53 and CREBBP), and mRNA-seq was performed on selected BCR-ABL1-like cases (n=41).
The genetic subgroups were divided into ETV6-RUNX1, TCF3-PBX1, hyperdiploid (>50 chromosomes), MLL rearrangements, BCR-ABL1, BCR-ABL1-like, ERG and other (cases with no known lesions). As expected, ETV6-RUNX1 and hyperdiploid ALL were less frequent in adolescents (4% and 11%, respectively) and adults (2% for both) than in childhood ALL (<16 years; 25% for both). In contrast, the frequency of BCR-ABL1-like ALL, a recently described subgroup in 10-15% of pediatric ALL associated with kinase-activating lesions and a poor outcome, was very frequent and increased with age (21% in adolescent, 25% in young adults), similar to cases with the classic BCR-ABL1 translocation (6% in adolescent, 22% in young adults).
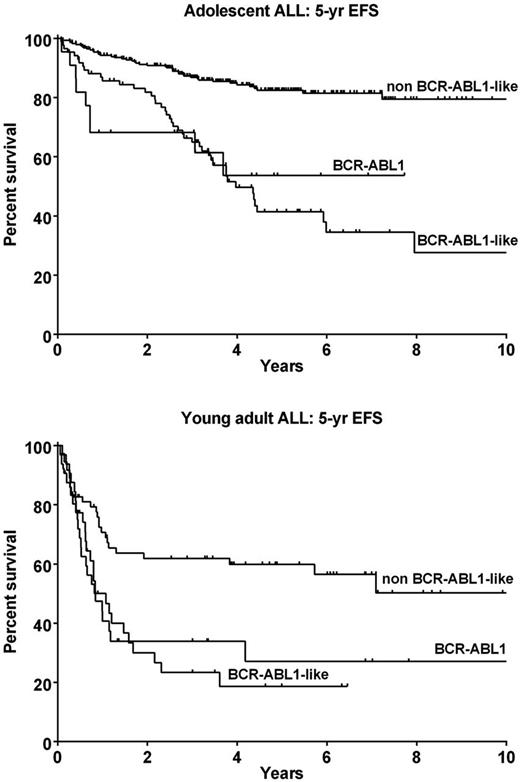
Notably, BCR-ABL1 and BCR-ABL1-like ALL patients presented with higher white blood counts at diagnosis compared to non BCR-ABL1-like ALL patients in both adolescents (117.6 and 76.8 vs 21.9 x109/L, p<0001), and young adults (72.6 and 94.1 vs 17.6 x109/L, p<0001). BCR-ABL1-like ALL patients were also more likely to be male compared to non BCR-ABL1-like ALL patients, with 74% vs 62% in adolescents (p<0.05; Fisher's exact test), and 81% vs 63% in young adults (p=0.07; Fisher's exact test). The outcome of BCR-ABL1 and BCR-ABL1-like ALL was markedly inferior to other ALL subtypes, with 5-year event free survival (EFS) rates of 53.7+18.3 and 40.0+7.1 vs 85.0±3.3 (p<0.0001) in adolescent cases, and 23.2±9.1 and 16.1±8.5 vs 57.9±8.0 (p=0.006) for young adults (Figure 1). IKZF1 alterations, a marker of poor outcome in pediatric ALL, were enriched in BCR-ABL1 and BCR-ABL1-like ALL cases (70% and 77%, respectively) compared to non BCR-ABL1-like patients (26%). Regardless of genetic subtype, the presence of an IKZF1 alteration correlated with inferior 5 year EFS in adolescent (60.3±6.0 vs 77.4±4.1; p=0.0015) and young adults (25.7±7.0 vs 52.7±6.4; p=0.0011).
We then sought to characterize the alterations activating kinase signaling in AYA BCR-ABL1-like ALL cases. As observed in pediatric ALL, approximately 55% of these cases harbored CRLF2 rearrangements. Using mRNA-seq we identified a variety of additional rearrangements involving the tyrosine kinase or cytokine receptor genes ABL1, ABL2, CSF1R, JAK2, EPOR or PDGFRB, with a marked enrichment of fusions involving JAK2 (6 different fusions in 9/20 cases sequenced), thus providing a rationale for the investigation of targeted therapies directed against these alterations. Collectively, the kinase-activating BCR-ABL1 and BCR-ABL1-like subtypes are associated with poor outcome and make up ∼25% of adolescent and ∼50% of young adult ALL patients. The identification of these patients at diagnosis will provide an opportunity to incorporate tyrosine kinase inhibitor treatment to current chemotherapeutic regimens, and significantly improve the treatment outcome for AYA ALL.
Hunger:Bristol Myers Squibb: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.