Key Points
We propose that megakaryopoiesis is regulated by the expression levels of the TPO receptor MPL and the associated tyrosine kinase JAK2.
This model could explain why suboptimal doses of JAK2 inhibitors can induce a paradoxical increase in platelet production.
Abstract
Megakaryopoiesis is a 2-step differentiation process, regulated by thrombopoietin (TPO), on binding to its cognate receptor myeloproliferative leukemia (MPL). This receptor associates with intracytoplasmic tyrosine kinases, essentially janus kinase 2 (JAK2), which regulates MPL stability and cell-surface expression, and mediates TPO-induced signal transduction. We demonstrate that JAK2 and MPL mediate TPO-induced proliferation arrest and megakaryocytic differentiation of the human megakaryoblastic leukemia cell line UT7-MPL. A decrease in JAK2 or MPL protein expression, and JAK2 chemical inhibition, suppress this antiproliferative action of TPO. The expression of JAK2 and MPL, which progressively increases along normal human megakaryopoiesis, is decreased in platelets of patients diagnosed with JAK2- or MPL-mutated essential thrombocytemia and primary myelofibrosis, 2 myeloproliferative neoplasms in which megakaryocytes (MKs) proliferate excessively. Finally, low doses of JAK2 chemical inhibitors are shown to induce a paradoxical increase in MK production, both in vitro and in vivo. We propose that JAK2 and MPL expression levels regulate megakaryocytic proliferation vs differentiation in both normal and pathological conditions, and that JAK2 chemical inhibitors could promote a paradoxical thrombocytosis when used at suboptimal doses.
Introduction
Megakaryopoiesis is the cellular process leading to platelet production from the differentiation of hematopoietic stem cells (HSC). It can be divided into a proliferative stage that generates megakaryocyte (MK) progenitors and precursors, and a maturation stage in which differentiating MKs proliferate no more. These 2 stages can be driven by thrombopoietin (TPO), which therefore exerts both proliferative and antiproliferative effects. TPO binding to its cognate receptor, myeloproliferative leukemia (MPL), activates multiple downstream signaling pathways.1-4 MPL being devoid of kinase activity, the receptor associates with intracytoplasmic tyrosine kinases, in particular janus kinase 2 (JAK2), for signal transduction.5-7 JAK2 is not only essential for TPO-induced signal transduction, but also for MPL stability and cell-surface expression.8
In essential thrombocytemia (ET) and primary myelofibrosis (PMF), 2 myeloproliferative neoplasms (MPNs), the abnormal accumulation of MKs suggests that these cells have escaped the proliferation arrest associated with terminal steps of differentiation.9-11 Mutations in JAK2 (JAK2V617F) and MPL are detected in ∼60% and ∼5% of PMF and ET, respectively.9 In addition, MPL is downregulated in MKs and platelets of PMF patients, and this decrease is a more controversial issue in ET.12-16
We have shown previously that TPO triggers MK proliferation arrest and promotes a differentiation-associated senescence through a strong mitogen-activated protein kinase (MAPK) signaling.17 In the present study, we show that TPO-induced proliferation vs differentiation depends on JAK2 and MPL protein levels. When 1 of these proteins is expressed at low level, TPO induces a weak signal that promotes cell proliferation. At higher JAK2 and MPL levels, TPO promotes cell-cycle arrest and MK differentiation. The modulation of MPL and JAK2 expression levels may regulate the 2 steps of normal megakaryopoiesis, and their downregulation may explain the abnormal proliferation of MKs in MPNs. This model could also explain the paradoxical increase in the platelet count induced by suboptimal doses of JAK2 chemical inhibitors.18
Materials and methods
Cell culture
The human megakaryoblastic UT7 cells expressing MPL (UT7-11oc1 to oc7 clones) were grown in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Cergy Pontoise, France). This medium was supplemented with 10% fetal bovine serum, antibiotics (100 IU/mL penicillin and 50 mg/mL streptomycin), and GM-CSF (5 ng/mL) or recombinant human TPO (hTPO) (10 ng/mL).
In vitro growth of MKs from CD34+ cells
Blood samples were obtained after informed consent in accordance with the Declaration of Helsinki. Approval was obtained from the Assistance Publique des Hôpitaux de Paris. CD34+ cells were isolated using immunomagnetic beads (Miltenyi Biotec, Paris, France) and grown in a serum-free medium, supplemented with hTPO (10 ng/mL; a generous gift from Kirin, Tokyo, Japan).
Lentiviral and retroviral vector construction and production of plasmids
Oligonucleotide JAK2 short hairpins (shRNA JAK2) listed in supplemental Table 1 (available on the Blood Web site) were synthesized (Eurogentec, Angers, France) and inserted into a pBlue Script containing the human H1 promoter. The H1-shJAK2 or H1-SCR (scramble control sequence, supplemental Table 1) cassette was inserted into a lentiviral vector (pRRLsin-PGK [Phosphoglycerate kinase]-eGFP-WPRE; Genethon, Evry, France). The cDNA encoding the human MPLWT was cloned into the bicistronic retroviral vector pMX-IRES-CD4. The cDNA encoding the human JAK2 (JAK2WT or JAKV617F) was cloned into a modified lentiviral vector pRRL, generated by replacing the promoter PGK with the promoter MND (governing JAK2 expression) followed by the promoter EF1a governing eGFP expression (TMJ-JAK2 vector, kindly provided by Dr Chloé James).
Production of retroviruses and lentiviruses
Vesicular stomatitis virus glycoprotein pseudotyped viral particles were produced into 293EBNA or 293T cells. Cells were infected with concentrated retrovirus or lentivirus supernatants for 2 hours at a multiplicity of infection of 10 and sorted by flow cytometry (FACS Vantage; BD Biosciences, Mountain View, CA) 48 hours later based on eGFP or CD4 expression.
Microarray analysis
The raw global gene expression values (from Affymetrix GeneChip) for 24-hour TPO-exposed UT7-11oc1-7 cells were processed with the robust multiarray analysis algorithm using BioConductor software, version 2.3.
Cell immunolabeling
UT7-11oc1-7 and CD34+ cells were rinsed in PBS, stained for 30 minutes at 4°C with anti-CD41–APC, anti-CD41-FITC, anti-CD42–PE, or an irrelevant mouse immunoglobulin G1 (IgG1) (BD Biosciences). MPL cell-surface expression was explored using the mouse monoclonal anti-MPL IgG2b, clone 1.7819 (a generous gift from Amgen, Thousand Oaks, CA), and indirect immunofluorescence with a FITC-goat anti-mouse Ig. Cell samples were analyzed by flow cytometry using FACS Vantage.
Immunoblotting
Immunoblotting was performed as described previously.17 Antibodies used: Actin (Sigma-Aldrich), JAK2 (no. 3230), phosphoJAK2 (no. 3776), phosphoSTAT5 (no. 9351), phosphoSTAT3 (no. 9134), phosphoSTAT1 (no. 9171), phosphoERK (no. 4377), ERK (no. 9102), p21CIP1 (no. 2947), PARP (no. 9542), caspase 3 (no. 9662) (Cell Signaling, Beverly, MA), p53 (no. SC-6243), CD42b (no. SC59051) (Santa Cruz Biotechnology, Heidelberg, Germany), TPOR/c-Mpl (EMD Millipore, Billerica, MA), and HSC70 (Stressgen). Proteins were detected using enhanced chemiluminescence (ECL; Pierce Perbio, Brebières, France) and were quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Quantitative real-time PCR
Total RNA was extracted using a Trizol RNA isolation kit (Invitrogen). Transcription into cDNA was performed using random hexamers and SuperScript II reverse transcriptase (Invitrogen). All PCR reactions used Taqman PCR Master Mix (Applied Biosystems, Foster City, CA) to a final volume of 20 μL. Each cDNA sample was analyzed in triplicate in the ABI PRISM 7900 Sequence Detection System (Applied Biosystems).
SA-β-galactosidase assay
Detection of SA-β-galactosidase activity was performed as described previously.17
Chemical inhibitors of JAK2 signaling
AZD1480 was kindly provided by AstraZeneca Pharmaceuticals (Waltham, MA). Stock solution was diluted in dimethylsulfoxide (DMSO; Sigma-Aldrich) and was then diluted in culture medium for use. Novartis (Basel, Switzerland) kindly provided ruxolitinib. Stock solution was diluted in water with 0.5% methocellulose and 0.05% tween 80 for use.
Treatment of C57BL/6 mice with ruxolitinib
C57BL/6 mice (Harlan France, Gannat, France) aged 8 to 9 weeks were treated with vehicle (0.5% methocellulose, 0.05% Tween 80) or ruxolitinib (INCB018424) twice daily by oral gavage (20 or 60 mg/kg body weight/day). Blood samples were collected after 5 days of treatment. Hematologic parameters were measured using an automated counter (MS9; Schloessing Melet, Cergy Pontoise, France). Animal experiments were performed in accordance with guidelines established by the Institutional Animal Committee.
Statistical test
Student t test was used in all experiments and was considered significant if P < .05.
Results
Derivation of UT7-MPL clones that escape TPO-induced cellular proliferation arrest
The human megakaryoblastic cell line UT7, transduced to express stably the TPO receptor MPL, has been largely used to study megakaryocytic differentiation.20,20-22 We have previously described the UT7-MPL clone named UT7-11oc1 in which TPO induces a proliferation arrest and a MK phenotype with senescence markers. These cells proliferate under GM-CSF exposure.17 From the UT7-11oc1 clone cultured in the presence of TPO, we derived the UT7-11oc2 subclone that escapes proliferation arrest (Figure 1A). This clone proliferates in the presence of TPO (Figure 1A) as well as in the presence of GM-CSF (Figure 1B). Morphologic changes (Figure 1C), SA-β-galactosidase activity (supplemental Figure 1A), expression of CD41 (Figure 1D), and p21CIP1 protein expression (Figure 1E and supplemental Figure 1B) observed in TPO-treated UT7-11oc1 cells are decreased or are no more observed in TPO-treated UT7-11oc2 cells. In addition, TPO-induced activation of the MAPK pathway is decreased in UT7-11oc2 cells (Figure 1E and supplemental Figure 1B). These results led us to use UT7-11oc2 cells to explore how cells can escape TPO-induced proliferation arrest.
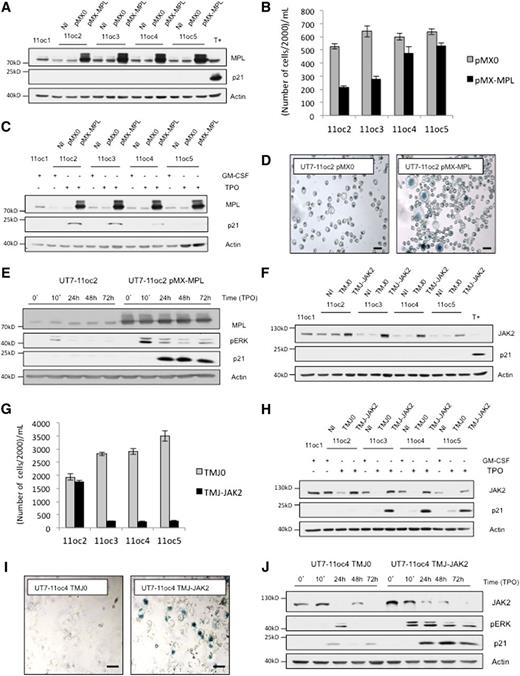
UT7-11oc2-7: clones derived from UT7-MPL (UT7-11oc1) cells escaping TPO-induced cellular proliferation arrest. Characterization of UT7-11oc2-7 clones according to MPL/JAK2 protein expression. We derived clones named UT7-11oc2-7 by culturing UT7-11oc1 cells in the presence of TPO. (A) Resurgence of cellular proliferation after a midterm (D10-D14) culture of UT7-11oc1 cells with TPO. Cells escaping TPO-induced proliferation arrest were named UT7-11oc2 (first clone derived). Viable cells were counted using Trypan blue exclusion. (B) UT7-11oc2 cells were cultured in the presence of either GM-CSF or TPO, and cell proliferation was compared with that of UT7-11oc1 cells exposed to TPO. (C) May Grünwald Giemsa staining in TPO-treated cells. (D) CD41 expression measured by flow cytometry in TPO-treated cells. (E) Differences in cell signaling, notably activation of MAPK pathway, and p21CIP1 expression for UT7-11oc1 and UT7-11oc2 cells. After a 12-hour cytokine starvation, UT7-11oc1 and UT7-11oc2 cells were restimulated by TPO during the indicated times, and cell signaling was studied by immunoblotting. (F) Clusterization of UT7-11oc2-7 clones according to TPO-induced gene expression profile. After a 12-hour cytokine starvation, UT7-11oc1 and UT7-11oc2-7 cells were stimulated with TPO for 24 hours. TPO-induced gene expression for each derived clone (UT7-11oc2-7)—relative to that of UT7-11oc1 cells—was determined by microarray analysis, and gene expression profiles obtained for the different UT7-11oc2-7 clones were compared with each other. (G) UT7-11oc1 cells and UT7-11oc2-7 clones were cultured with GM-CSF and MPL and JAK2 protein expression analyzed by immunoblotting. (H) MPL cell-surface expression was analyzed by flow cytometry for cells cultured in the presence of GM-CSF. Mean fluorescence intensity was 1190 for UT7-11oc1 cells vs 743 for UT7-11oc2 cells. Bars: (C) 50 μm. Error bars represent mean ± standard deviation of 3 independent experiments.
UT7-11oc2-7: clones derived from UT7-MPL (UT7-11oc1) cells escaping TPO-induced cellular proliferation arrest. Characterization of UT7-11oc2-7 clones according to MPL/JAK2 protein expression. We derived clones named UT7-11oc2-7 by culturing UT7-11oc1 cells in the presence of TPO. (A) Resurgence of cellular proliferation after a midterm (D10-D14) culture of UT7-11oc1 cells with TPO. Cells escaping TPO-induced proliferation arrest were named UT7-11oc2 (first clone derived). Viable cells were counted using Trypan blue exclusion. (B) UT7-11oc2 cells were cultured in the presence of either GM-CSF or TPO, and cell proliferation was compared with that of UT7-11oc1 cells exposed to TPO. (C) May Grünwald Giemsa staining in TPO-treated cells. (D) CD41 expression measured by flow cytometry in TPO-treated cells. (E) Differences in cell signaling, notably activation of MAPK pathway, and p21CIP1 expression for UT7-11oc1 and UT7-11oc2 cells. After a 12-hour cytokine starvation, UT7-11oc1 and UT7-11oc2 cells were restimulated by TPO during the indicated times, and cell signaling was studied by immunoblotting. (F) Clusterization of UT7-11oc2-7 clones according to TPO-induced gene expression profile. After a 12-hour cytokine starvation, UT7-11oc1 and UT7-11oc2-7 cells were stimulated with TPO for 24 hours. TPO-induced gene expression for each derived clone (UT7-11oc2-7)—relative to that of UT7-11oc1 cells—was determined by microarray analysis, and gene expression profiles obtained for the different UT7-11oc2-7 clones were compared with each other. (G) UT7-11oc1 cells and UT7-11oc2-7 clones were cultured with GM-CSF and MPL and JAK2 protein expression analyzed by immunoblotting. (H) MPL cell-surface expression was analyzed by flow cytometry for cells cultured in the presence of GM-CSF. Mean fluorescence intensity was 1190 for UT7-11oc1 cells vs 743 for UT7-11oc2 cells. Bars: (C) 50 μm. Error bars represent mean ± standard deviation of 3 independent experiments.
JAK2 or MPL expression is decreased in UT7-MPL cells that evade TPO-induced cellular proliferation arrest
Using the same method, we derived 5 other clones (UT7-11oc3-7) that escape the antiproliferative action of TPO (supplemental Figure 1C). All these clones remain dependent on cytokines for their growth, as evidenced by cellular apoptosis induced by a 24-hour cytokine starvation (supplemental Figure 1D). We compared gene expression after a 24-hour exposure to TPO in UT7-11oc1 and the 6 UT7-MPL subclones UT7-11oc2-7. Nonsupervised analysis separated 2 groups of proliferative clones, UT7-11oc2 and UT7-11oc3 on one hand, and the other clones on the other, possibly indicating distinct evasion mechanisms (Figure 1F). One of the downregulated genes was JAK2. Because MPL and JAK2 are key actors in TPO-induced biological response, we studied the level of MPL and JAK2 proteins in these different clones. We observed a decrease in MPL expression in UT7-11oc2 cells and a decrease in JAK2 protein level in UT7-11oc3-7 cells, compared with UT7-11oc1 (Figure 1G). These decreases correlated with a decrease in the corresponding mRNA expression (supplemental Figure 1E). MPL cell-surface expression, evaluated by flow cytometry, was decreased in all studied UT7-11oc2-5 clones as compared with UT7-11oc1 cells (Figure 1H and supplemental Figure 1F). This was confirmed by considering the upper band on MPL immunoblots, which corresponds to the mature, glycosylated form of the receptor (Figure 1G). The decreased membrane expression of MPL in UT7-11oc3-5 clones was in accordance with the role of JAK2 in MPL stability and trafficking.8
MPL and JAK2 expression levels determine the response of UT7-MPL cells to TPO
To demonstrate directly that MPL cell-surface expression was the major determinant in the cellular response to TPO, we sorted UT7-11oc1 and UT7-11oc2 cells depending on their MPL membrane expression (supplemental Figure 2A for UT7-11oc1). However, fluorescence intensity for UT7-11oc2 MPLhigh cells peaked below that of UT7-1oc1 MPLlow cells (622 vs 750, not shown). With this strategy, we could not modulate the proliferative phenotype under TPO for both clones (supplemental Figure 2B). Thus we used another strategy, which objective was to increase MPL or JAK2 expression, by retrovirus or lentivirus transduction, in UT7-11oc2 to 11oc5 clones. Of note, whereas this approach led to a main increase in MPL protein expression (Figure 2A), MPL cell-surface expression remained in the same order of magnitude for infected cells (supplemental Figure 2C). The increased expression of MPL did not promote p21CIP1 protein expression in response to GM-CSF in any clone (Figure 2A). In UT7-11oc2 and UT7-11oc3 but not in UT7-11oc4 and 5, MPL-increased expression restored the cell-cycle arrest in response to TPO (Figure 2B). This effect was associated with an increase in p21CIP1 expression (Figure 2C), morphologic changes (not shown), and SA-β-galactosidase activity (Figure 2D). MPL overexpression also increased ERK phosphorylation after TPO treatment (see clone UT7-11oc2 on Figure 2E). Similar to MPL, the increase in JAK2 expression did not promote p21CIP1 protein expression in response to GM-CSF in any clone (Figure 2F). After JAK2 overexpression, TPO induced a decrease in cell proliferation (Figure 2G), an increase in p21CIP1 protein expression (Figure 2H), morphologic modifications (not shown), and SA-β-galactosidase activity (Figure 2I) in all clones except UT7-11oc2. JAK2 overexpression also increased ERK phosphorylation after TPO treatment (see UT7-11oc4 clone, Figure 2J). Altogether, MPL and JAK2 expression levels determined the MAPK cellular response to TPO and the antiproliferative effect of the cytokine.
An increase in MPL or JAK2 expression, by viral transduction, according to the decreased protein expression, reinduces a proliferation arrest in the presence of TPO. (A) UT7-11oc2-5 clones were transduced with either empty retroviral vector (pMX0) or the vector encoding for MPL (pMX-MPL) and were cultured in the presence of GM-CSF. MPL and p21CIP1 protein expression were determined by immunoblotting. T+ corresponds to UT7-11oc1 cells exposed to TPO. (B) Cellular proliferation in presence of TPO. Cells were seeded at 20 000 cells/mL (D0) and counted at D5. (C) p21CIP1 expression was determined for cells exposed to TPO for 3 days, by immunoblotting. (D) SA-β-galactosidase staining in TPO-treated cells at D5. (E) After a 12 h cytokine starvation, UT7-11oc2 cells transduced with either the MPL-expression or control vector were stimulated with TPO. MPL, p21CIP1 expression and ERK phosphorylation status were then analyzed by immunoblotting. (F) UT7-11oc2-5 clones were transduced with either empty lentiviral vector (TMJ0) or the vector encoding for JAK2 (TMJ-JAK2) and cultured in presence of GM-CSF. MPL and p21CIP1 protein expressions were determined by immunoblotting. T+ corresponds to UT7-11oc1 cells exposed to TPO. (G) Cellular proliferation in the presence of TPO. Cells were seeded at 20 000 cells/mL and were counted at D7. (H) p21CIP1 expression was determined by immunoblotting for cells exposed to TPO for 4 days. (I) SA-β-galactosidase staining in TPO-treated cells at D5. (J) After a 12-hour cytokine starvation, UT7-11oc4 cells transduced with either the JAK2-expression or control vector were stimulated with TPO. MPL, p21CIP1 expression, and ERK phosphorylation status were then analyzed by immunoblotting. Bars: (D,I) 50 μm. Error bars represent standard deviations of 2 independent experiments. NI, noninfected cells.
An increase in MPL or JAK2 expression, by viral transduction, according to the decreased protein expression, reinduces a proliferation arrest in the presence of TPO. (A) UT7-11oc2-5 clones were transduced with either empty retroviral vector (pMX0) or the vector encoding for MPL (pMX-MPL) and were cultured in the presence of GM-CSF. MPL and p21CIP1 protein expression were determined by immunoblotting. T+ corresponds to UT7-11oc1 cells exposed to TPO. (B) Cellular proliferation in presence of TPO. Cells were seeded at 20 000 cells/mL (D0) and counted at D5. (C) p21CIP1 expression was determined for cells exposed to TPO for 3 days, by immunoblotting. (D) SA-β-galactosidase staining in TPO-treated cells at D5. (E) After a 12 h cytokine starvation, UT7-11oc2 cells transduced with either the MPL-expression or control vector were stimulated with TPO. MPL, p21CIP1 expression and ERK phosphorylation status were then analyzed by immunoblotting. (F) UT7-11oc2-5 clones were transduced with either empty lentiviral vector (TMJ0) or the vector encoding for JAK2 (TMJ-JAK2) and cultured in presence of GM-CSF. MPL and p21CIP1 protein expressions were determined by immunoblotting. T+ corresponds to UT7-11oc1 cells exposed to TPO. (G) Cellular proliferation in the presence of TPO. Cells were seeded at 20 000 cells/mL and were counted at D7. (H) p21CIP1 expression was determined by immunoblotting for cells exposed to TPO for 4 days. (I) SA-β-galactosidase staining in TPO-treated cells at D5. (J) After a 12-hour cytokine starvation, UT7-11oc4 cells transduced with either the JAK2-expression or control vector were stimulated with TPO. MPL, p21CIP1 expression, and ERK phosphorylation status were then analyzed by immunoblotting. Bars: (D,I) 50 μm. Error bars represent standard deviations of 2 independent experiments. NI, noninfected cells.
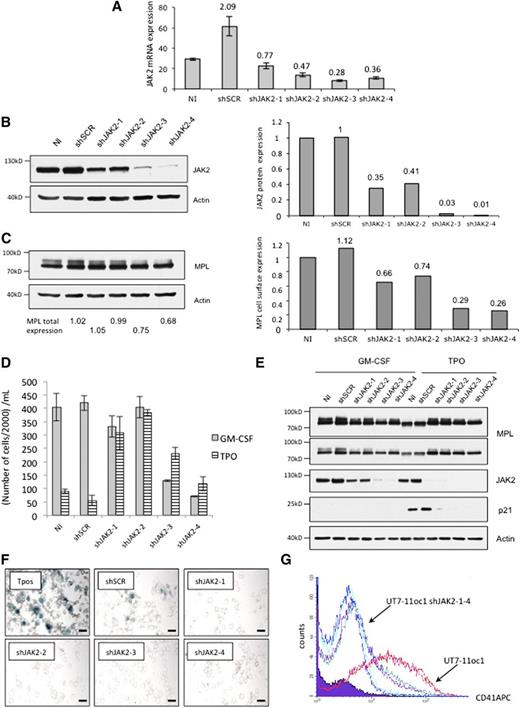
To explore further this hypothesis, that is, the proliferation arrest requests a strong signaling, we studied the effects of various TPO concentrations on UT7-11oc1 cells. At 0.1 ng/mL, TPO promoted UT7-11oc1 cell proliferation, whereas higher concentrations did not (supplemental Figure 3A). A proliferative effect of highest concentrations of TPO was restored by combination with the JAK2 chemical inhibitor AZD1480 at 1 μM (supplemental Figure 3B), a concentration that inhibits cell signaling (supplemental Figure 3C). Of note, higher concentrations of AZD1480 (>5 μM) inhibited cell proliferation, as expected (supplemental Figure 3D). Restoration of TPO-induced cell proliferation was associated with the inhibition of morphologic changes (supplemental Figure 3E). We also knocked down JAK2 in UT7-11oc1 cells using 4 lentiviral shRNAs whose efficacy was checked at the mRNA (Figure 3A) and protein (Figure 3B) levels. As expected, given the role of JAK2 in MPL cell trafficking, the decreased expression of JAK2 (Figure 3B) correlated with a decreased membrane expression of MPL (Figure 3C). A slight decrease in JAK2 expression with shJAK2-1 and shJAK2-2 promoted cell proliferation in response to TPO (Figure 3D) while inhibiting TPO-induced p21CIP1 expression (Figure 3E), morphologic changes (Figure 3F), and CD41 expression (Figure 3G) without altering growth under GM-CSF (Figure 3D). The important decrease in JAK2 expression by shJAK2-3 and −4 markedly inhibited the growth under GM-CSF but still increased cell growth under TPO in comparison with the control cell line (Figure 3D). Altogether, the response of UT7-MPL cells to TPO depends on the MP/JAK2 expression level, a higher expression being requested for TPO to arrest cell proliferation and to promote cell differentiation (see model on Figure 4A). According to this demonstration, a decrease in JAK2 expression can be sufficient to escape the antiproliferative effect of TPO.
JAK2 shRNA expression in UT7-11oc1 cells restores cellular proliferation and inhibits the megakaryocytic phenotype in the presence of TPO. (A) shRNA lentiviral transductions of UT7-11oc1 cells were efficient to inhibit JAK2 mRNA expression. UT7-11oc1 cells were infected with shJAK2-1-4, cultured in the presence of GM-CSF and JAK2 mRNA expression (relative to HPRT) evaluated by quantitative real-time PCR. (B) shRNA lentiviral transductions of UT7-11oc1 cells were efficient to inhibit JAK2 protein expression. UT7-11oc1 cells were infected with shJAK2-1-4, cultured in the presence of GM-CSF and JAK2 protein expression (relative to actin) evaluated by immunoblotting. (C) Parallel to JAK2 expression, total and cell-surface MPL expression was determined by immunoblotting. The shRNA-expressing cells were seeded at 25 000 cells/mL and were studied at D4. The shJAK2-1-4-expressing cells were characterized by a cellular proliferation (D), the lack of p21CIP1 protein expression (E), morphologic changes and SA-β-galactosidase staining (F), and by a decreased megakaryocytic markers (CD41) expression (G) in the presence of TPO. Bars: (E) 50 μm. Error bars represent mean ± standard deviation of 2 independent experiments.
JAK2 shRNA expression in UT7-11oc1 cells restores cellular proliferation and inhibits the megakaryocytic phenotype in the presence of TPO. (A) shRNA lentiviral transductions of UT7-11oc1 cells were efficient to inhibit JAK2 mRNA expression. UT7-11oc1 cells were infected with shJAK2-1-4, cultured in the presence of GM-CSF and JAK2 mRNA expression (relative to HPRT) evaluated by quantitative real-time PCR. (B) shRNA lentiviral transductions of UT7-11oc1 cells were efficient to inhibit JAK2 protein expression. UT7-11oc1 cells were infected with shJAK2-1-4, cultured in the presence of GM-CSF and JAK2 protein expression (relative to actin) evaluated by immunoblotting. (C) Parallel to JAK2 expression, total and cell-surface MPL expression was determined by immunoblotting. The shRNA-expressing cells were seeded at 25 000 cells/mL and were studied at D4. The shJAK2-1-4-expressing cells were characterized by a cellular proliferation (D), the lack of p21CIP1 protein expression (E), morphologic changes and SA-β-galactosidase staining (F), and by a decreased megakaryocytic markers (CD41) expression (G) in the presence of TPO. Bars: (E) 50 μm. Error bars represent mean ± standard deviation of 2 independent experiments.
Cellular response to TPO depends on MPL/JAK2 expression. A model for normal and pathological megakaryopoiesis regulation by JAK2 and MPL expression levels. (A) The cell line model: TPO-induced cellular response depends on MPL/JAK2 protein expression in the UT7-MPL cell line model. A decrease in the expression of MPL at the cell surface can be induced by a decrease in either MPL (UT7-11oc2) or JAK2 (UT7-11oc3-7 and UT7-11oc1 shJAK2) protein expression. (B) We propose that during normal megakaryopoiesis, MPL/JAK2 expression could reach a threshold at which the signal induced by TPO switches from proliferation to cell-cycle arrest and terminal maturation. According to this model, each event inducing a decrease in JAK2 or MPL expression/signaling could extend the proliferative stage of megakaryopoiesis, resulting in an amplification of the progenitor cells’ compartment. This mechanism could be complementary, with the autonomous signaling in immature cells induced by mutations such as JAK2V617F or MPLW515 mutations. The situation that could occur in MPNs is shown (red lines).
Cellular response to TPO depends on MPL/JAK2 expression. A model for normal and pathological megakaryopoiesis regulation by JAK2 and MPL expression levels. (A) The cell line model: TPO-induced cellular response depends on MPL/JAK2 protein expression in the UT7-MPL cell line model. A decrease in the expression of MPL at the cell surface can be induced by a decrease in either MPL (UT7-11oc2) or JAK2 (UT7-11oc3-7 and UT7-11oc1 shJAK2) protein expression. (B) We propose that during normal megakaryopoiesis, MPL/JAK2 expression could reach a threshold at which the signal induced by TPO switches from proliferation to cell-cycle arrest and terminal maturation. According to this model, each event inducing a decrease in JAK2 or MPL expression/signaling could extend the proliferative stage of megakaryopoiesis, resulting in an amplification of the progenitor cells’ compartment. This mechanism could be complementary, with the autonomous signaling in immature cells induced by mutations such as JAK2V617F or MPLW515 mutations. The situation that could occur in MPNs is shown (red lines).
MPL and JAK2 expression levels could determine the response of normal MKs to TPO
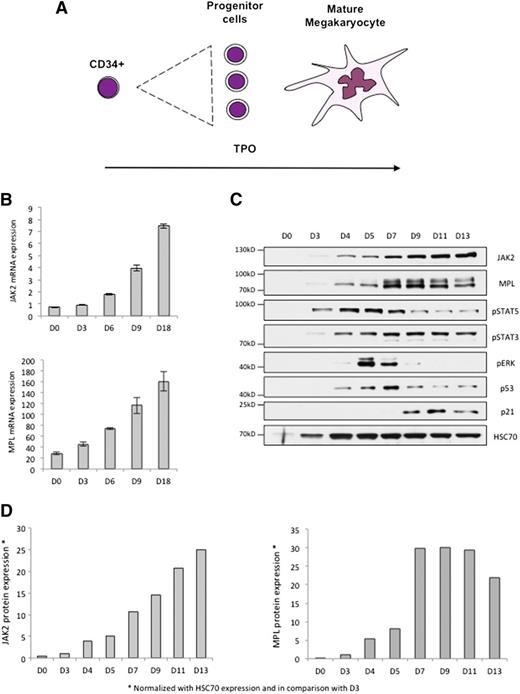
Megakaryopoiesis is a dynamic process with a proliferation step followed by a maturation step, both induced by TPO (Figure 5A). We cultured CD34+ cells in a serum-free medium in the presence of TPO to trigger their MK differentiation, and we observed a differentiation-associated increase in MPL and JAK2 mRNA expression levels (Figure 5B). JAK2 protein expression increased with differentiation, whereas that of MPL remained stable or decreased at the end of maturation (Figure 5C-D). Analysis of cell signaling along megakaryocytic differentiation identified an activation of STAT5 that peaked at day 5, a sustained phosphorylation of STAT3, and a phosphorylation of ERK1/2 that peaked between day 5 and day 7, preceding p21CIP1 expression (Figure 5C). These observations—compared with the results obtained in the UT7-MPL clones—suggest that the differentiation-associated increase in MPL and JAK2 expression promotes a switch from proliferation to the differentiation step of TPO-driven megakaryopoiesis.
MPL and JAK2 levels are upregulated during megakaryocytic differentiation. The increase in MPL/JAK2 protein expression could regulate megakaryopoiesis. (A) Megakaryopoiesis can be regulated by a single cytokine, namely TPO, which exerts both proliferative and antiproliferative effects. (B) Human CD34+ cells from cytapheresis were cultured in a serum-free medium (MSS) in the presence of TPO at 10 ng/mL. MPL and JAK2 mRNA expression (relative to HPRT) was determined by Taqman during megakaryopoiesis at days 0, 3, 6, 9, and 18. (C) MPL, JAK2, p53, p21CIP1 expression and STAT5, STAT3, ERK phosphorylation status were analyzed by immunoblotting during human megakaryopoiesis at days 0, 3, 4, 5, 7, 9, 11, and 13. (D) Quantification of JAK2 and MPL protein expression (normalized with HSC70 expression and in comparison with D3) during megakaryopoiesis in vitro. Error bars represent mean ± standard deviation.
MPL and JAK2 levels are upregulated during megakaryocytic differentiation. The increase in MPL/JAK2 protein expression could regulate megakaryopoiesis. (A) Megakaryopoiesis can be regulated by a single cytokine, namely TPO, which exerts both proliferative and antiproliferative effects. (B) Human CD34+ cells from cytapheresis were cultured in a serum-free medium (MSS) in the presence of TPO at 10 ng/mL. MPL and JAK2 mRNA expression (relative to HPRT) was determined by Taqman during megakaryopoiesis at days 0, 3, 6, 9, and 18. (C) MPL, JAK2, p53, p21CIP1 expression and STAT5, STAT3, ERK phosphorylation status were analyzed by immunoblotting during human megakaryopoiesis at days 0, 3, 4, 5, 7, 9, 11, and 13. (D) Quantification of JAK2 and MPL protein expression (normalized with HSC70 expression and in comparison with D3) during megakaryopoiesis in vitro. Error bars represent mean ± standard deviation.
Altered expression of MPL and JAK2 in platelets of PMF and ET patients
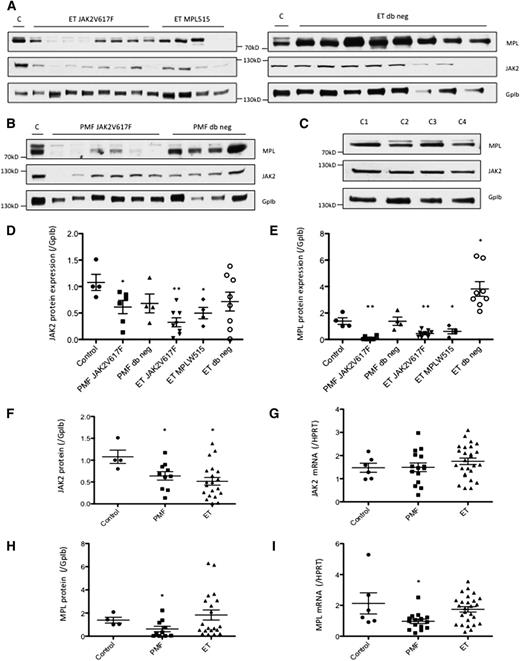
According to the model in which high JAK2 and MPL levels induce an antiproliferative signal, it is expected that the expression level of these 2 proteins is decreased in PMF and ET. Accordingly, immunoblot analyses of these proteins in platelets show a decrease in their expression in the context of JAK2V617F and MPLW515 mutations in PMF and ET samples (Figure 6A-C). Quantification of the JAK2 protein expression confirms a significant decrease in platelets of PMF JAK2V617F (P < .05), ET JAK2V617F (P < .01), and ET MPLW515 (P < .05), compared with platelets from healthy donors (Figure 6D). Similarly, quantification of MPL protein expression indicates a significant decrease in platelets of PMF JAK2V617F (P < .01), ET JAK2V617F (P < .01), and ET MPLW515 (P < .05) compared with platelets from healthy donors (Figure 6E). Surprisingly, JAK2 and MPL expression was not decreased in patients without JAK2V617F and MPLW515L/K, and an increase in MPL expression was even observed in platelets of ET patients without any mutation in JAK2 and MPL (P < .05) (Figure 6E). The decreased expression of JAK2 protein in PMF and ET (Figure 6F) was independent of any change at the mRNA level, suggesting a posttranslational mechanism (Figure 6G). The mechanism of MPL-decreased expression (Figure 6H) remained unclear as it was associated to a decreased mRNA level in PMF (P < .05) but not in ET (nonsignificant) (Figure 6I). To explore the mechanism underlying the decrease in JAK2 protein expression in the context of a JAK2V617F mutation, we expressed the mutant protein in UT7-MPL cells. We had noticed that, in UT7-11oc1 cells, TPO specifically induced a decrease in JAK2 protein expression (supplemental Figure 4A), following its phosphorylation (supplemental Figure 4B), without any decrease in JAK2 mRNA (supplemental Figure 4C). This effect was not observed with other cytokines, including GM-CSF and erythropoietin (supplemental Figure 4A-B,E). Cell pretreatment with the proteasome inhibitor lactacystin before TPO treatment partially restored JAK2 protein expression, arguing for a proteasomal degradation of JAK2 on TPO exposure (supplemental Figure 4D). Finally, whereas overexpression of wild-type JAK2 in UT7-11oc1 cells induced the expected increase in JAK2 protein expression, that of JAK2V617F protein induced a paradoxical decrease in JAK2 protein level (supplemental Figure 4F), suggesting that JAK2V617F could promote the degradation of the wild-type protein.
MPL/JAK2 mRNA and protein expression in platelets of MPN patients. Platelets were collected from patients diagnosed with PMF or ET, or from healthy donors (Controls C1 to C4). MPL/JAK2 protein expression was evaluated by immunoblotting for ET patients (A) and PMF patients (B) and was compared with normal controls (C). MPL/JAK2 protein expression, normalized with that of GpIb, was quantified using imageJ. (D) Quantification of JAK2 protein expression in platelets of MPN patients according to the MPL/JAK2 mutational status. (E) Quantification of MPL protein expression in platelets of MPN patients according to their MPL and JAK2 mutational status. (F) JAK2 protein expression and (G) JAK2 mRNA expression were determined for PMF an ET samples. (H) MPL protein expression and (I) MPL mRNA expression were determined for PMF and ET samples. Error bars indicate mean ± standard error of the mean. *P < .05; **P < .01.
MPL/JAK2 mRNA and protein expression in platelets of MPN patients. Platelets were collected from patients diagnosed with PMF or ET, or from healthy donors (Controls C1 to C4). MPL/JAK2 protein expression was evaluated by immunoblotting for ET patients (A) and PMF patients (B) and was compared with normal controls (C). MPL/JAK2 protein expression, normalized with that of GpIb, was quantified using imageJ. (D) Quantification of JAK2 protein expression in platelets of MPN patients according to the MPL/JAK2 mutational status. (E) Quantification of MPL protein expression in platelets of MPN patients according to their MPL and JAK2 mutational status. (F) JAK2 protein expression and (G) JAK2 mRNA expression were determined for PMF an ET samples. (H) MPL protein expression and (I) MPL mRNA expression were determined for PMF and ET samples. Error bars indicate mean ± standard error of the mean. *P < .05; **P < .01.
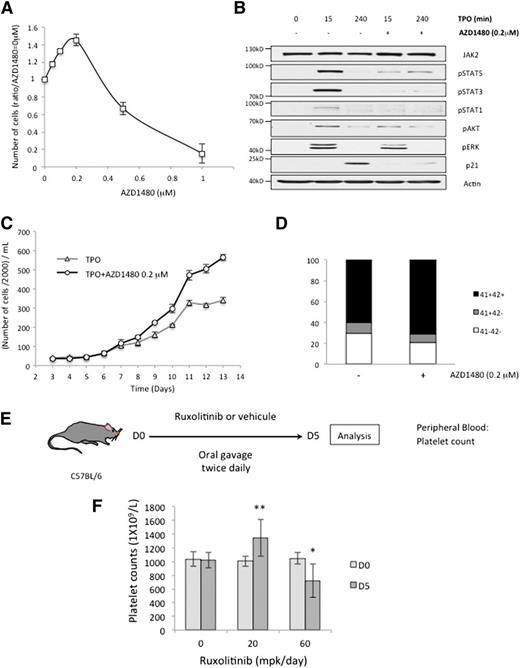
JAK2 inhibitors at suboptimal doses increase MKs production in vitro and in vivo
We then studied the effect of JAK2 chemical inhibitors on megakaryocytopoiesis. A low concentration of the JAK2 inhibitor AZD1480 (0.2 µM) induced an increase in MK production from cord blood CD34+ cells exposed to TPO (Figure 7A). At this concentration, AZD1480 inhibits TPO-induced cell signaling (Figure 7B) and stimulates the MK production from adult CD34+ cells exposed to TPO (Figure 7C), without affecting differentiation marker expression (Figure 7D), or the percentage of platelets-generating MKs (not shown). Higher concentrations of AZD1480 (eg, 1 µM) completely inhibited cell proliferation and differentiation (Figure 7A). Finally, we treated C57BL/6 mice twice daily by oral gavage with vehicule (0.5% methocellulose, 0.05% tween 80) or the JAK2 inhibitor ruxolitinib at 20 or 60 mg per kg of body weight (mpk) per day (Figure 7E). As expected, given the essential role of JAK2 in TPO-induced signal transduction, ruxolitinib at 60 mpk induced a decrease in the platelet number (798 ± 226.109 vs 1018 ± 112.109) (Figure 7F, P < .05). However, a lower dose of ruxolitinib (20 mpk) induced a paradoxical and significant increase in the platelet count (1298 ± 241.109) (Figure 7F, P < .01).
Low doses of JAK2 chemical inhibitors induce a paradoxical increase in MKs production both in vitro and in vivo. (A) The effect of AZD1480, a JAK2 chemical inhibitor, at different concentrations on cellular proliferation of CD34+ cells from cord blood cultured in MSS with TPO at 10 ng/mL. Cells were counted at D8. (B) CD34+ cells were cultured with TPO for 6 days. After a 12-hour cytokine starvation, these cells were restimulated with 10 ng/mL TPO with or without AZD1480 pretreatment, at 0.2 μM during 2 hours. We determined the efficacy of AZD1480 on cell signaling by immunoblotting. (C) CD34+ cells from cytapheresis were cultured in MSS with TPO, with or without AZD1480 at a concentration of 0.2 μM. Cellular proliferation was evaluated until D13 and CD41 and CD42 expression was determined by flow cytometry at D8 (D). (E) C57BL/6 mice were treated with different doses of ruxolitinib, a JAK2 inhibitor used in clinics (20 and 60 mg/kg of body weight per day) or vehicule (methocellulose 0.5%, tween 80 0.05%) by oral gavage, twice daily. Blood samples were analyzed after 5 days of treatment. (F) Platelet counts for C57BL/6 mice treated during 5 days with vehicule (n = 9), ruxolitinib at 20 mpk (n = 12), or 60 mpk (n = 9). Error bars represent mean ± standard deviation of 3 (A,F) or 2 (C) independent experiments. *P < .05; **P < .01.
Low doses of JAK2 chemical inhibitors induce a paradoxical increase in MKs production both in vitro and in vivo. (A) The effect of AZD1480, a JAK2 chemical inhibitor, at different concentrations on cellular proliferation of CD34+ cells from cord blood cultured in MSS with TPO at 10 ng/mL. Cells were counted at D8. (B) CD34+ cells were cultured with TPO for 6 days. After a 12-hour cytokine starvation, these cells were restimulated with 10 ng/mL TPO with or without AZD1480 pretreatment, at 0.2 μM during 2 hours. We determined the efficacy of AZD1480 on cell signaling by immunoblotting. (C) CD34+ cells from cytapheresis were cultured in MSS with TPO, with or without AZD1480 at a concentration of 0.2 μM. Cellular proliferation was evaluated until D13 and CD41 and CD42 expression was determined by flow cytometry at D8 (D). (E) C57BL/6 mice were treated with different doses of ruxolitinib, a JAK2 inhibitor used in clinics (20 and 60 mg/kg of body weight per day) or vehicule (methocellulose 0.5%, tween 80 0.05%) by oral gavage, twice daily. Blood samples were analyzed after 5 days of treatment. (F) Platelet counts for C57BL/6 mice treated during 5 days with vehicule (n = 9), ruxolitinib at 20 mpk (n = 12), or 60 mpk (n = 9). Error bars represent mean ± standard deviation of 3 (A,F) or 2 (C) independent experiments. *P < .05; **P < .01.
Discussion
Cellular response to cytokines depends on the nature, duration, strength, and cellular localization of the intracellular signals that are generated. It is generally assumed that physiologically, a low-intensity signal promotes only cell survival, a weak or moderate signal induces cell proliferation, whereas a strong signal induces cell-cycle arrest by different ways including differentiation, senescence, and apoptosis.23,23-27 Physiological MK differentiation has been shown to request a strong activation of the MAPK pathway.21,22,28 Here, we propose that TPO, the central cytokine in this process, induces a weak signal in MK progenitors, which promotes their proliferation, while inducing a strong signal in more mature cells, which induces their terminal differentiation. Alteration in this dual effect of TPO, as a result of a deregulated JAK2 and MPL expression, may account for the abnormal MK proliferation that characterizes some MPNs.
The proof of concept of this dual regulation was obtained in the UT7-MPL cell line in which the ability to proliferate in response to TPO was acquired in subclones wherein the expression of MPL or JAK2 protein was decreased. MPL/JAK2 expression determined whether TPO triggered cell-cycle arrest with acquisition of an MK phenotype (higher MPL/JAK2 expression), or whether it promoted cell proliferation without differentiation (lower MPL/JAK2 expression). TPO-induced cell-cycle arrest was associated with a strong activation of the MAPK pathway in accordance with previous studies,21,22 whereas the MAPK signal was weaker when TPO was inducing proliferation. Altogether, the ability of some UT7-MPL clones exposed to TPO to proliferate depended on JAK2 and MPL protein expression, which determines downstream cell signaling intensity. The dose-dependent effect of TPO on cell proliferation and the ability of the chemical JAK2 inhibitor AZD1480 at suboptimal doses to promote proliferation of cells that might otherwise undergo differentiation further argued for the proposed hypothesis. Although flow cytometry analysis of MPL membrane expression identified a threshold in TPO-induced cellular response, we cannot rule out the selection of another genetic event that accounts for the differential response of the studied subclones to TPO. Nevertheless, we demonstrated that shRNA-mediated decrease in JAK2 protein expression, which correlates with a decrease in MPL cell-surface expression, can be sufficient to escape the antiproliferative action of TPO.
MPL and JAK2 protein expression was found to increase regularly along normal MK differentiation. Based on the observations made in the UT7-MPL model, we propose that the MPL/JAK2 expression could reach a threshold at which the signal induced by TPO switches from proliferation to cell-cycle arrest and terminal maturation. According to this model, every event leading to a decrease in MPL or JAK2 expression or in signaling intensity may extend the proliferative stage and might induce an amplification of the progenitor cell compartment in MPNs. Accordingly, a thrombocytosis is observed in transgenic mice expressing reduced levels of MPL,29,30 and a decrease in MPL expression was observed in platelets and MKs of patients with MPNs, with either PMF or ET.13-15 This decrease in MPL expression was obvious in patients harboring a JAK2V617F or a MPLW515 mutation, whereas MPL expression was increased in ET patients without any of these mutations. As we were not able to evaluate reliably the cell-surface expression of MPL in these patients, we cannot exclude a decreased expression in their MK progenitors related to a trafficking defect. In any event, these results may explain the controversial reports of MPL expression in ET.12-16 The mechanisms leading to an MPL expression decrease could depend on the disease as MPL mRNA expression was decreased in PMF whereas it was normal in ET, suggesting that a transcriptional mechanism could operate in PMF. The frequent mutations in epigenetic regulators identified in this disorder could participate in MPL deregulation.31,32
JAK2 protein expression has not been often studied in MPNs. We detected a decrease in JAK2 protein expression in platelets of MPN patients, correlated with the presence of a JAK2V617F or a MPLW515 mutation, and without any change in mRNA expression, suggesting a posttranscriptional mechanism that may be related to the constitutive activation and phosphorylation of JAK2. Given the role of JAK2 in MPL stability and traffic,8 JAK2-decreased expression could participate in the decreased cell-surface expression of the TPO receptor. In the context of heterozygosity, observed in about half of ET, JAK2V617F may also induce the degradation of the wild-type JAK2, consecutively decreasing MPL membrane expression. This mechanism could occur in addition to the recently reported proteasomal degradation following MPL ubiquitination by JAK2V617F.33 Here, we provide some evidence that JAK2V617F can induce a decrease in total JAK2 expression in UT7-MPL cell line. The reduced cell signaling intensity during the first stage of megakaryopoiesis (resulting from a decreased MPL/JAK2 expression) would then extend the proliferative stage, allowing the progenitor cells to perform additional mitosis, leading to increased MK production.
The discrepancies between MPNs and congenital amegakaryocytic thrombocytopenia could be explained by the role of MPL in the HSC compartment. Gain-of-function MPL mutations seen in MPNs may confer an advantage to the HSC (survival, self-renewal, exit from quiescence) by activating signaling, whereas loss-of-function MPL mutations described in congenital amegakaryocytic thrombocytopenia, which impede TPO signaling, may result in a defect in HSC and hematopoietic cell production, resulting in amegakaryocytosis.
The proposed model suggests that weak signaling may be involved in the abnormal MK proliferation. In favor of this hypothesis, low concentrations of the JAK2 inhibitor AZD1480 induced an increase in the MK number generated in vitro in the presence of TPO, without any change in MK markers and proplatelet formation, which is a result in apparent contradiction with the thrombocytopenia described in clinical trials.34 As expected, higher concentrations of the JAK2 inhibitor induced cell death. Thus, a suboptimal inhibition of JAK2 could possibly increase platelet production. Accordingly, whereas ruxolinitib, a JAK2 inhibitor used in clinics, induced thrombocytopenia in mice treated with high doses, the drug paradoxically increased the platelet count when used at suboptimal doses. An increased platelet count was also reported in mice treated with MRLB-11055, a highly potent inhibitor of JAK2,35 whereas an increased platelet count was observed in some PMF patients treated with lower doses of ruxolitinib.18
In conclusion, we propose that the expression level of JAK2 and MPL regulates the switch from proliferation to differentiation in the megakaryocytic lineage (Figure 4B). According to this model, the downregulation of JAK2 and MPL combined with the autonomous signaling resulting from mutations may extend the proliferative stage of megakaryopoiesis and enhance MK production. Similarly, an incomplete inhibition of JAK2, as obtained with suboptimal doses of currently developed inhibitors, may induce a paradoxical thrombocytosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr C. James for viral vector construction, Philippe Dessen, Justine Guégan, and Thomas Robert for gene-array analysis, INSERM, the Foundation Aging Research Centre, and the Association Laurette Fugain for support (R.B.), and the Ligue Nationale Contre le Cancer for support (E.S. and W.V. teams). The authors thank Kerin Brewery (Tokyo, Japan) for the generous gift of recombinant TPO.
Authorship
Contribution: R.B. designed research, performed research, analyzed data, and wrote the paper; D.R.-W. designed research, performed research, and analyzed data; C.T., H.A., C.L., F.P., C.W., P.R., Y.L., and J.-B.M. performed research and analyzed data; S.G., E.S., and W.V. designed research, analyzed data, and wrote the paper; and S.N.C. analyzed data and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Correspondence: Stéphane Giraudier, INSERM UMR 1009, Institut Gustave Roussy 114, Rue Edouard Vaillant, 94805 Villejuif, France; e-mail: stephane.giraudier@hmn.aphp.fr.