Abstract
Background
Intensive chemotherapy is associated with poor tolerability and high early mortality in the treatment of AML in older patients. Lower intensity approaches using hypomethylating agents have been developed in patients with lower bone marrow (BM) blast counts, but more effective therapies are needed, particularly in those with higher BM blasts. Cladribine, which itself may have hypomethylating properties, has single-agent activity in AML. Cladribine has also been shown to improve survival in AML in combination with standard-dose araC (Holowiecki JCO 2012).
Methods
We developed a low-intensity, prolonged-maintenance treatment protocol studying the combination of cladribine and low-dose araC (LDAC) alternating with decitabine in patients aged ≥ 60. Patients with adequate organ function and newly diagnosed AML (except APL), including secondary- (s-AML) and therapy-related AML (t-AML), and high risk MDS were eligible. Induction consisted of cladribine 5 mg/m2 IV over 30 minutes on days 1-5 followed by araC 20mg SQ BID on days 1-10. Consolidation/maintenance consisted of 2 cycles of cladribine 5 mg/m2 IV over 30 minutes on days 1-3 + araC 20 mg SQ BID on days 1-10 alternating with 2 cycles of decitabine 20 mg/m2 on days 1-5, for a maximum of 18 cycles. One cycle was 4 weeks and up to 2 cycles of induction were allowed.
Results
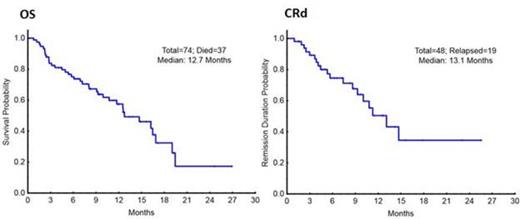
A total of 74 patients have been enrolled with a median age of 69 years (range, 49-85), including 33 pts (45%) ≥ age 70. 41 pts (55%) had secondary- (s-AML) or therapy-related AML (t-AML) and 14 pts (19%) had therapy for an antecedent hematologic disorder. Patient characteristics are listed in table 1. Of the 74 pts evaluable for response, there were 42 CR (57%), 6 CRp (8%), and 3 PR (4%) for an overall response rate of 69% (51/74). 43 patients (58%) had BM blasts ≥ 30%, and they had a CR/CRp rate of 67%. Of the 43 patients who achieved a CR/CRp and had minimal residual disease testing (MRD) by flow cytometry, 28 patients (65%), achieved MRD negativity at the time of CR or later. AML mutation screening was performed prior to treatment. Ten of 10 patients (100%) with FLT3-mutated AML and 13/13 (100%) pts with an NPM1 mutation achieved a CR/CRp. With a median follow-up of 11+ months, the median OS is 12.7 months and the median CR duration is 13.1 months. (Figure 1) The 1-year OS estimate is 57%. The regimen was very well tolerated, with 1% 4-week mortality. There were no treatment-related grade 3/4 non-hematologic adverse events (AEs). Most common non-hematologic AEs were elevated bilirubin, nausea/vomiting, rash/itching, diarrhea and mucositis.
Conclusion
The low intensity program of cladribine + LDAC alternating with decitabine is a highly effective, well-tolerated ambulatory regimen for older patients, including those with unfavorable-risk features. Patient samples are being analyzed for methylation changes on treatment.
Patient Characteristics (N=74)
| Characteristic . | N (%) . |
|---|---|
| Median age [Range] | 69 [49-85] |
| Cytogenetics | |
| Diploid | 21 (28) |
| Adverse | 30 (41) |
| -5/-7 | 23 (31) |
| Complex without -5/-7 | 7 (9) |
| Misc. other | 17 (23) |
| Insufficient | 6 (8) |
| Mutations | |
| NPM1 | 13/69 (19) |
| FLT3-ITD | 8/69 (12) |
| RAS | 15/69 (22) |
| Median BM Blast [Range] | 32 [8-95] |
| Median WBC [Range] | 2.5 [0.5-72.3] |
| Median Platelets [Range] | 40 [4-772] |
| Median Peripheral Blasts [Range] | 6 [0-88] |
| Median LDH [Range] | 742 [301-8425] |
| Median Creatinine [Range] | 0.91 [0.46-1.94] |
| Median Bilirubin [Range] | 0.6 [0.2-2] |
| Characteristic . | N (%) . |
|---|---|
| Median age [Range] | 69 [49-85] |
| Cytogenetics | |
| Diploid | 21 (28) |
| Adverse | 30 (41) |
| -5/-7 | 23 (31) |
| Complex without -5/-7 | 7 (9) |
| Misc. other | 17 (23) |
| Insufficient | 6 (8) |
| Mutations | |
| NPM1 | 13/69 (19) |
| FLT3-ITD | 8/69 (12) |
| RAS | 15/69 (22) |
| Median BM Blast [Range] | 32 [8-95] |
| Median WBC [Range] | 2.5 [0.5-72.3] |
| Median Platelets [Range] | 40 [4-772] |
| Median Peripheral Blasts [Range] | 6 [0-88] |
| Median LDH [Range] | 742 [301-8425] |
| Median Creatinine [Range] | 0.91 [0.46-1.94] |
| Median Bilirubin [Range] | 0.6 [0.2-2] |
Kantarjian:ARIAD, Pfizer, Amgen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.