Key Points
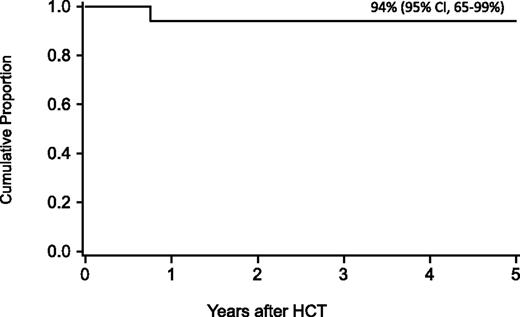
With no prior opportunistic infections/transfusions, 5-year survival after alternative donor HCT with TBI 300 cGy, CY, FLU, and ATG was 94%.
Today, most FA patients with standard risk disease are cured of their bone marrow failure by HCT even without an HLA-matched sibling donor.
Abstract
Historically, alternative donor hematopoietic cell transplantation (HCT) for Fanconi anemia (FA) patients resulted in excessive morbidity and mortality. To improve outcomes, we made sequential changes to the HCT conditioning regimen. A total of 130 FA patients (median age, 9.0 years; range, 1-48) underwent alternative donor HCT at the University of Minnesota between 1995 and 2012. All patients received cyclophosphamide (CY), single fraction total body irradiation (TBI), and antithymocyte globulin (ATG) with or without fludarabine (FLU), followed by T-cell–depleted bone marrow or unmanipulated umbilical cord blood transplantation. The addition of FLU enhanced engraftment 3-fold. The incidence of grades 2-4 acute and chronic graft-versus-host disease was 20% and 10%, respectively. Severe toxicity was highest in patients >10 years of age or those with a history of opportunistic infections or transfusions before HCT. Mortality was lowest in patients without a history of opportunistic infection or transfusions and who received conditioning with TBI 300 cGy, CY, FLU, and ATG. These patients had a probability of survival of 94% at 5 years. Alternative donor HCT is now associated with excellent survival for patients without prior opportunistic infections or transfusions and should be considered for all FA patients after the onset of marrow failure. These studies were registered at http://www.clinicaltrials.gov as NCT00005898, NCT00167206, and NCT00352976.
Introduction
Fanconi anemia (FA) is a rare genetically and phenotypically heterogeneous inherited disorder characterized by congenital malformations, progressive bone marrow (BM) failure, and marked predisposition to malignancy.1 Hematological abnormalities occur in at least 90% of FA patients at a median onset of 7 years,2,3 and allogeneic hematopoietic cell transplantation (HCT) is the only proven potential curative therapy.4 Most FA patients do not have an HLA-identical unaffected sibling donor and therefore require an alternative (HLA-matched related or unrelated) donor. Early experiences with alternative donor HCT for the treatment of the hematological complications of FA were discouraging, with long-term survival rates of approximately 30%.5,6 Poor outcomes were often the result of graft failure in 25% to 30% patients, graft-versus-host disease (GVHD) in 50% to 70% patients, excessive regimen-related toxicities, and opportunistic infections.5,6
To improve outcomes, we made sequential changes to the HCT conditioning regimen in a series of 4 prospective clinical trials. The aim of this analysis was to identify patient-, treatment-, and graft-related factors associated with favorable outcomes.
Patient and methods
Study design
This is an analysis of all patients with FA undergoing alternative donor HCT at the University of Minnesota between February 1995 and June 2012. Clinical and laboratory data were prospectively collected in the University of Minnesota Blood and Marrow Transplant Database and analyzed as of April 2014. All patients and/or guardians signed institutional review board–approved informed consent in accordance with the Declaration of Helsinki.
Patients
Eligible patients had FA with severe marrow failure (defined as having either hemoglobin <8 g/dL, platelet count <20 × 109/L, and/or absolute neutrophil count <5 × 108/L), advanced myelodysplastic syndrome (MDS with ≥5% blasts), or acute leukemia.7 Patients were excluded from transplant if organ function was inadequate (ie, left ventricular ejection fraction <45%, any liver function test >5× normal, oxygen saturation <92% in room air), for poor performance status (Karnofsky <70% or Lansky <50%), active uncontrolled infection, or a history of squamous cell carcinoma within 2 years.
Transplant procedure
All patients received a conditioning regimen containing a single fraction total body irradiation (TBI), cyclophosphamide (CY) 10 mg/kg per day IV with divided dose mesna 10 mg/kg per day IV for 4 days, and equine antithymocyte globulin (ATG), with cyclosporine A as the principal agent for GVHD prophylaxis. Modifications were made over time including the addition of fludarabine (FLU) 35 mg/m2 per day IV ×4 days (1999), the use of thymic shielding8 during TBI (2003), a reduction of TBI to 300 cGy (2006), and the use of mycophenolate mofetil instead of methylprednisolone for GVHD prophylaxis along with cyclosporine A (2010), as shown in Table 1.
Marrow from a 7/8 allele level HLA-matched related or 7 to 8/8 allele level HLA-matched unrelated donor was ex vivo T-cell–depleted with an add back of T cells to achieve a fixed graft T-cell dose of 1 × 105 CD3/kg recipient. Unmanipulated 4 to 6/6 HLA-matched unrelated umbilical cord blood (UCB) was used based on standard matching criteria.9
In all groups, TBI was administered in a single fraction delivered with anteroposterior and posteroanterior fields at extended distance (dose rate was 26 cGy/min) on day –6 before HCT. The TBI dose was prescribed at the midplane of the patient at the midpelvis. Lung compensators were designed to maintain prescribed dose (±5%) to the lungs. For thymic shielding, a contrast enhanced treatment planning computed tomography scan was performed to locate the thymus. 5 half value layer Cerrobend blocks were fabricated and placed to block the thymus with a 1-cm margin from both the anterior and posterior TBI fields.
Patients were hospitalized in single rooms with high-efficiency particulate air filtration with positive pressure until neutrophil recovery. Patients received antibiotic prophylaxis at least until engraftment and antifungal antimicrobials until at least day 100. Patients received acyclovir prophylaxis if they were seropositive for herpes simplex virus and/or cytomegalovirus (CMV). Oral trimethoprim-sulfamethoxazole was given for Pneumocystis prophylaxis after engraftment for 1 year. Broad-spectrum IV antibacterial and as-indicated additional antifungal and/or antiviral antimicrobials were administered for fevers. CMV-seronegative recipients received CMV-safe (CMV-seronegative or filtered) blood products. Additionally, patients received antifungal prophylaxis, including itraconazole (2000-2003) and later voriconazole (2003-2012) 1 month before admission. All patients received granulocyte colony-stimulating factor (G-CSF, Neupogen) 5 µg/kg per day IV from the day after transplant until neutrophil recovery. CMV reactivation was monitored weekly until at least day 100 following transplant and preemptively treated with ganciclovir or foscarnet.
End point definitions
Time to neutrophil recovery was the first of 3 consecutive days on which the absolute neutrophil count (ANC) was ≥0.5 × 109/L. Primary graft failure was defined as failure to achieve an ANC of 0.5 × 109/L by day 42 and secondary graft failure as an ANC <0.5 × 109/L for 3 consecutive days or 0% donor DNA by molecular analysis having previously achieved an ANC of ≥0.5 × 109/L. Time to platelet recovery was the first of 3 consecutive days (or laboratory measurements) on which the platelet count was >20 × 109/L without transfusions for 7 days before the first measurement. BM aspirations and biopsies were performed routinely at 21, 100, and 180 days and 1 and 2 years after HCT and more frequently if graft failure or relapse was suspected. Donor origin of reconstituted cells was documented by molecular analysis. GVHD was graded by standard criteria.10
Infection data were collected prospectively and then audited for completeness and accuracy by retrospective review of the outpatient and inpatient records of patients transplanted from 1999 and beyond, as described previously.11 Infections were categorized as being either serious viral infections including CMV, Epstein-Barr virus, adenovirus, influenza, parainfluenza and respiratory syncytial virus, fungal infections caused by mold or yeast infections, or bacterial infections. Infections caused by Clostridium difficile or herpes zoster were not included. MDS or acute leukemia relapse was disease recurrence at any site and supported by cytogenetic and molecular analyses. Survival was time from transplant to death from any cause. Transplant-related mortality was death due to any cause other than recurrent MDS or leukemia.
Statistical analysis
Kaplan-Meier curves were used to estimate the probability of survival.12 The log-rank test was used to compare outcomes between groups. Cox regression was used to examine the independent effect of factors on survival.13 Factors considered in regression models included preparative therapy (no FLU + TBI [450 or 600 cGy] vs FLU + TBI 450 cGy vs FLU + TBI 300 cGy), donor type (HLA-mismatched related BM vs HLA-matched unrelated-donor BM vs HLA-mismatched unrelated BM vs UCB), age (<10 vs ≥10), disease status (aplastic anemia/early MDS [<5% blasts] vs late MDS [≥5% blasts]/acute myeloid leukemia), clonal cytogenetic abnormalities (yes vs no), number of malformations (<3 vs ≥3),6 gender (male vs female), gender donor/recipient match (match vs mismatch), performance score at baseline (Lansky/Karnofsky score 60-80 vs 90-100), prior opportunistic infection (yes vs no), CMV serostatus (recipient negative/donor negative vs recipient negative/donor positive vs recipient positive), prior transfusions (none vs any), diepoxybutane (DEB) T-cell mosaicism (presence of resistant cells in ≥10% vs < 10%), DEB sensitivity (mean chromosomal breaks per cell), time-dependent acute GVHD, and prior androgen therapy (yes vs no).
All factors were tested for violations of the proportional hazards assumption using Martingale residuals. Neutrophil recovery, platelet recovery, acute and chronic GVHD, regimen-related toxicities, opportunistic infections, and mortality were estimated with cumulative incidence treating nonevent deaths as a competing risk.14 Fine and Gray regression was used to examine the independent effect of factors for these end points.15 The adjusted overall survival and engraftment curves of different types of conditioning in Figures 1 and 2 were computed as average estimates of the pooled sample, weighted by the proportions of the significant variables in the regression models.16,17 Multivariate analysis of the incidence of opportunistic infections was completed by using a Cox regression–type analysis to calculate the relative risks. However, the correlation of multiple events within each subject was taken into account by an appropriate correction to the variance estimate, and an appropriate risk set was defined for each infection. The model that performed this task was the conditional model of Prentice et al for multiple infections of a similar type.18 Logistic regression analysis was used to determine risk factors for grade 4 or 5 toxicities using National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Analyses were performed with SAS 9.3 (SAS Institute, Cary, NC) and R 3.0.2 (R Project for Statistical Computing, http://www.r-project.org/).
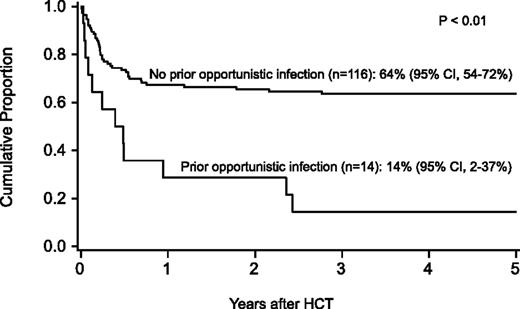
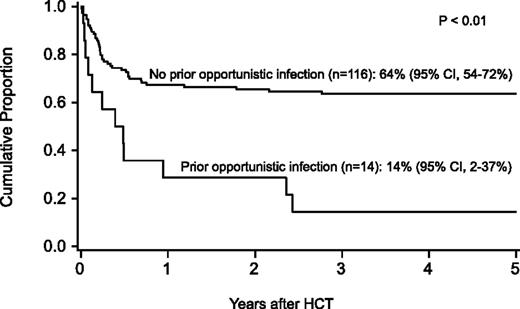
Probability of survival after HCT by history of opportunistic infection before HCT.
Probability of survival after HCT by history of opportunistic infection before HCT.
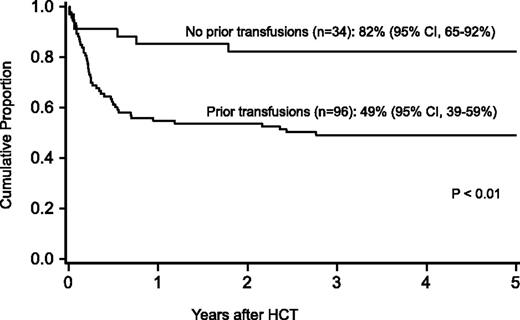
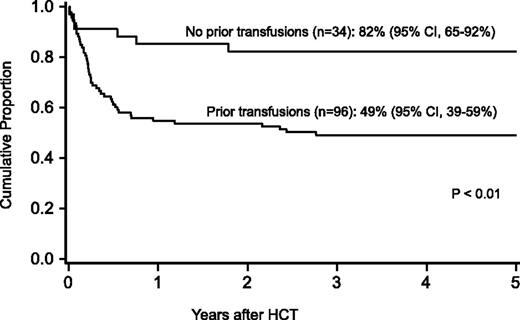
Probability of survival after HCT by history of transfusions before HCT.
Results
Patient and donor characteristics
One hundred and thirty patients with FA, median age 9.0 years (range, 1-48) with 19 (15%) >18 years), underwent alternative donor HCT (Table 2). The population reflects the expected distribution of FA gene mutations (68% FA complementation group A [FANCA], 20% FANCC, 1% FANCD1, 1% FANCE, 2% FANCF, and 8% FANCG of the 100 patients with known mutations). DEB mosaicism (ie, presence of ≥10% DEB-resistant cells) was present in 36% patients. Before HCT, patients were treated for opportunistic infections11 (11%) or cytopenias with blood products (74%), hematopoietic growth factors (48%), or androgens (38%). Median follow-up was 7.7 years (range, 1.8-18.8).
Survival
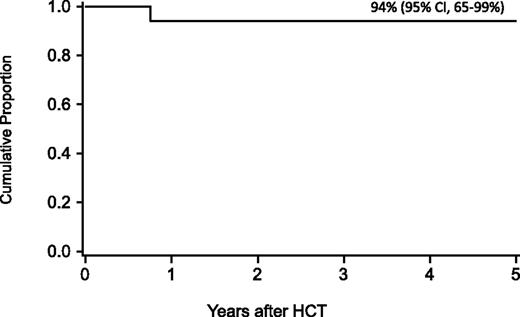
For the entire cohort, the probability of survival at 1 year was 63% (95% confidence interval [CI], 54-71), at 5 years 58% (95% CI, 49-59), and at 10 years 57% (95% CI, 47-65-%). In regression analysis, recipients of FLU-containing regimens had a lower risk of mortality at 5 years (Table 3), especially those who received FLU and TBI 300 cGy (relative risk [RR], 0.1; 95% CI, 0.03-0.2; P < .01). Higher mortality at 5 years was associated with older age (10-17 years, RR, 2.2; 95% CI 1.1-4.5; P = .03; 18+ years, RR, 2.7; 95% CI, 1.2-5.8; P = .01), prior opportunistic infection (RR, 3.5; 95% CI, 1.6-7.5; P < .01), positive recipient CMV serostatus (RR, 2.3; 95% CI, 1.2-4.5; P = .02) and a trend toward higher mortality for patients receiving transfusions of packed red blood cells or platelets before the beginning of the conditioning regimen (RR, 2.3; 95% CI, 1.0-5.6; P = .06) (Figures 1 and 2). Factors that did not influence the risk of survival included gender, number of malformations, DEB mosaicism, complementation group, androgen or G-CSF use before transplant, disease status, clonal cytogenetic abnormalities, performance score, and renal function before transplant. For 17 patients (including 2 patients >18 years of age) without a prior history of opportunistic infection or transfusions who received a conditioning regimen of TBI 300 cGy, CY, FLU, and ATG, probability of survival at 5 years was 94% (95% CI, 65-99; Figure 3).
Probability of survival after HCT in patients without a prior history of opportunistic infection or transfusions who received conditioning with TBI 300 cGy and thymic shielding, CY, FLU, and ATG.
Probability of survival after HCT in patients without a prior history of opportunistic infection or transfusions who received conditioning with TBI 300 cGy and thymic shielding, CY, FLU, and ATG.
Given that the trials were conducted consecutively, the period of transplant and trials had a statistically collinear relationship, making it not possible to include both factors in the regression model. Notwithstanding, we investigated year of transplant within trials that spanned a timeframe of 4 to 8 years. The relative risk of mortality did not change over time within each trial, being 1.0 (95% CI, 0.5-1.8; P = .98) for trial 1 (FLU + TBI), 0.9 (95% CI, 0.7-1.1; P = .24) for trials 2 and 3 (FLU + TBI 450 cGy), and 0.8 (95% CI, 0.5-1.1; P = .18) for trial 4 (FLU + TBI 300 cGy).
Causes of death were graft failure (n = 5), GVHD (n = 10), regimen-related toxicity (n = 13), opportunistic infection (n = 17), relapse (n = 4), posttransplant lymphoproliferative disease (n = 2), and new malignancy (n = 2).
Hematopoietic recovery
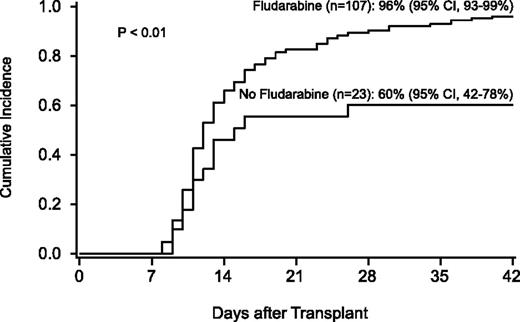
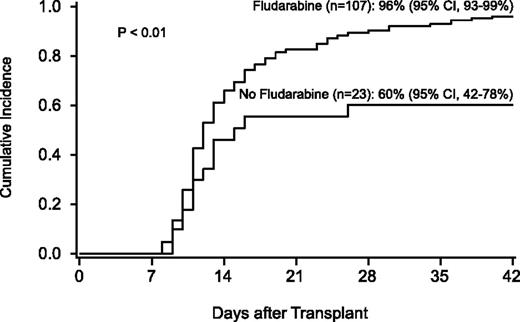
The incidence of neutrophil recovery was 90% (95% CI, 84-95) at a median of 12 days (range, 8-40). Factors associated with neutrophil recovery were use of FLU in the conditioning regimen and hematopoietic stem cell source (Table 4; Figure 4). The use of UCB was associated with a lower likelihood of neutrophil recovery (RR, 0.2; 95% CI, 0.1-0.4; P < .01) compared with HLA-matched unrelated-donor BM, but was strongly influenced by the conditioning regimen. For 46 recipients of the FLU/TBI 300 cGy–based conditioning regimen, neutrophil recovery was similar in recipients of BM and UCB, occurring in 28 of 29 T-cell–depleted BM recipients and in 15 of 17 UCB recipients. Chimerism data from BM were available after 1999. All patients who achieved neutrophil recovery had complete marrow chimerism by day 100.
Probability of neutrophil recovery by use of fludarabine in the conditioning regimen.
Probability of neutrophil recovery by use of fludarabine in the conditioning regimen.
Platelet recovery data were not collected for patients transplanted before 2000, restricting this analysis to 105 patients. For these patients, all of whom received a FLU-containing regimen, the incidence of platelet recovery by 6 months was 77% (95% CI, 65-89) at a median of 40.5 days (range, 16-238). In regression analysis, factors associated with platelet recovery were use of androgens before transplant and hematopoietic stem cell source. Patients who received androgens before transplant (RR, 0.5; 95% CI, 0.3-0.9; P = .02) or transplanted with UCB (RR, 0.5; 95% CI, 0.3-0.8; P = .01) were less likely to achieve platelet recovery by 6 months. Importantly, no other factors, including DEB mosaicism, TBI dose, use of thymic shielding, or cell dose, adversely affect neutrophil or platelet recovery engraftment.
GVHD
The incidence of grade 2-4 and grade 3-4 acute GVHD was 20% (95% CI, 13-27%) and 9% (95% CI, 4-14), respectively. In regression analysis, the only factor associated with acute GVHD was hematopoietic stem cell source. Recipients of unrelated donor 7/8 HLA-matched T-cell–depleted BM (RR, 2.8; 95% CI, 1.1-7.4; P = .03) or 4-6/6 HLA-matched UCB (RR, 2.9; 95% CI, 1.2-7.0; P = .02) were more likely to have grade 2-4 acute GVHD than recipients of HLA-matched T-cell–depleted BM. Similarly, recipients of unrelated-donor HLA-mismatched T-cell–depleted BM (RR, 4.5; 95% CI, 1.1-19.1; P = .04) or UCB (RR, 5.5; 95% CI, 1.4-22.1; P = .02) were more likely to have severe (grade 3-4) acute GVHD than recipients of HLA-matched T-cell–depleted BM. The overall incidence of chronic GVHD was 10% (95% CI, 5-15) at 2 years. In regression analysis, the only factor associated with chronic GVHD was the use of androgens before transplant (RR, 3.4; 95% CI, 1.1-10.6; P = .03). No other factor was associated with developing chronic GVHD, although there was a trend toward higher chronic GVHD in patients with prior acute GVHD (RR, 3.8; 95% CI, 0.8-17.9; P = .09).
Regimen-related toxicity
Detailed toxicity data were available for patients transplanted after 1999 (Table 5). For these patients, the overall incidence of any nonhematological severe (grade 4 or 5) toxicity was 43% (95% CI, 34-52). In regression analysis, factors associated with the development of severe regimen-related toxicities were older age (≥10 years [odds ratio [OR], 2.9; 95% CI, 1.2-7.0; P = .02]), prior opportunistic infections (OR, 5.7; 95% CI, 1.1-29.7; P = .04), and history of any prior transfusions (OR, 5.0; 95% CI, 1.7-15.3; P < .01). No other factor, including preparative therapy or graft source, showed significant association with severe regimen-related toxicities.
Opportunistic infections
One hundred and seventy-six severe opportunistic infection events were reported in the 105 patients transplanted after 1999. Forty-five patients did not develop any opportunistic infections. Among severe opportunistic infections, 58% were bacterial, 31% were viral, and 11% were fungal. Among the disseminated fungal infections, yeast infections by Candida (n = 12) occurred more often than mold infections caused by Aspergillus (n = 7).
The frequency of all opportunistic infections per patient was higher in patients with a history of opportunistic infection before transplant (RR, 1.8; 95% CI, 1.3-2.5; P < .01) or who were CMV-seropositive (RR, 2.0; 95% CI, 1.4-2.7; P < .01). Stem cell source was not a significant factor for overall risk of opportunistic infections. Risk factors for serious bacterial infections included prior opportunistic infections (RR, 1.9; 95% CI, 1.3-3.5; P = .04), prior transfusions (RR, 1.9; 95% CI, 1.1-3.4; P = .03), and HLA-mismatched BM (RR, 2.9; 95% CI, 1.2-7.1; P = .02). Invasive fungal infections were more likely in patients who were CMV-seropositive (RR, 6.7; 95% CI, 1.6-29.0; P = .01) or received TBI ≥450 (RR, 3.7; 95% CI, 1.0-14.3; P = .06). Viral infections were more common in patients who were CMV-seropositive (RR, 4.0; 95% CI, 1.8-8.8; P < .01).
Relapse and new malignancies
Two of 10 patients with advanced MDS or acute leukemia relapsed at 32 days and 175 days after transplant for an incidence of 20% (95% CI, 1-45). Twelve patients had Epstein-Barr virus reactivation, with 8 developing posttransplant lymphoproliferative disease (PTLD), of whom 7 received unrelated donor BM and one UCB as grafts. Four patients died of PTLD or complications of therapy. Three patients developed non–lympho-hematological malignancies after transplant for an overall incidence of 3% (95% CI, 0-6). All 3 had squamous cell carcinoma (mouth in 2, lung in 1) at 1, 12.4, and 14 years after transplant. None of these patients had a history of acute or chronic GVHD.
Discussion
This is the largest single-center experience of alternative donor HCT for the treatment of the hematological manifestations of FA. Establishing the optimal approach to achieve engraftment without excessive toxicity has been particularly challenging for FA patients because of their inherent DNA repair defect and intolerance to conventional doses of chemotherapy and radiation.19-21 In 1984, based upon in vitro laboratory studies that confirmed hypersensitivity of FA cells to chemotherapy19,20 and radiation,22 Gluckman et al proposed the use of low-dose chemotherapy and unfractionated radiation in conditioning regimens,21 which led to less toxicity and an improved yet suboptimal 2-year survival rate of 29%.5 Graft failure occurred in approximately 1/3 of FA patients,5,23 especially in those with T-cell mosaicism,23 suggesting that the presence of DEB-resistant T cells increased the risk of rejection. In 1999, FLU, an antimetabolite with profound immunosuppressive qualities, was added to the conditioning regimen, leading to higher rates of engraftment, even in patients with mosaicism. This beneficial effect of FLU on engraftment has also been reported by the Center for International Blood and Marrow Transplant Registry24 and the European Group for Blood and Marrow Transplantation,25 together establishing the importance of FLU in the conditioning regimens for FA patients undergoing alternative donor HCT. Importantly, this effect was sustained even when the TBI dose was decreased to 300 cGy.
Although the trials were conducted consecutively, we observed a similar risk of mortality within a given trial over time, suggesting that the conditioning regimen change with each trial was the primary factor responsible for improved outcomes in our cohort. Importantly, we have demonstrated that successful alternative donor HCT for FA patients can be achieved with less conditioning therapy, which is particularly pertinent for this patient population with DNA repair defects.
Thymic shielding was employed to minimize thymic damage from TBI to potentially hasten immune recovery.26-28 Importantly, there was no deleterious effect of thymic shielding on engraftment. Although there was a trend toward lower rates of opportunistic infections in patients who had thymic shielding, its role on enhancing immune recovery requires larger patient numbers.
Although UCB has historically been associated with inferior engraftment rates, the conditioning regimen, degree of HLA mismatch, and cell dose are key factors.29,30 Our results demonstrate that UCB is a suitable hematopoietic stem cell source in conjunction with our FLU- and TBI 300–based conditioning regimen, increasing the likelihood of identifying a suitable graft for most FA patients. New methods of ex vivo expansion are under investigation, potentially changing the pace of hematopoietic recovery and engraftment after UCB transplantation in the future. Despite an increased incidence of GVHD, it is encouraging that 1 antigen-mismatched related and unrelated T-cell–depleted marrow was not associated with higher mortality; however, the number of patients is limited to fully evaluate these stem cell sources.
Reducing the risk of GVHD is particularly important because the inherently high risk for malignancies, particularly squamous cell carcinomas of the head and neck, is magnified in FA patients who develop GVHD.25,31,32 The risk of acute and chronic GVHD rates was low in this cohort.24,25,32 Importantly, we observed a 3-fold increased risk of acute GVHD in recipients of T-cell–depleted HLA-mismatched BM or UCB compared with recipients of HLA-matched BM, demonstrating that HLA-matched BM is still the preferred alternative donor graft source. Additional strategies are needed to overcome GVHD in patients with HLA-mismatched BM or UCB donors.
Few patients in our series developed malignancies compared with other large series in the literature,25,32 which may be at least partly due to their low incidence of acute and GVHD. However, follow-up in our most recent cohort is insufficient for evaluating malignancy risk. Eight patients developed PTLD, all of whom received ATG, a known risk factor for developing PTLD.33,34 To potentially decrease the risk of PTLD, we have eliminated ATG in our current conditioning regimen for FA patients receiving alternative donor HCT.
Although age <10 years was associated with better outcomes, a finding in other disease groups undergoing HCT was that FA patients should only undergo HCT when necessary for severe marrow failure, advanced MDS, or acute leukemia.35-37 Additional prognostic factors observed by us and others include donor source, CMV serostatus, prior transfusions, and opportunistic infections but not number of malformations.6,24,25,29
Over the past 20 years, historical obstacles of excessive regimen-related toxicities, immunological graft rejection, and GVHD have been circumvented with lower dose conditioning, the addition of FLU and graft T-cell depletion. As a result, heightened surveillance is required to minimize transplant risk factors such as opportunistic infections, need for transfusions, or advanced MDS/leukemia. Although there is renewed interest in the use of androgens in this patient population, there are deleterious side effects that should be considered because they may adversely affect transplant outcomes.38-40 Alternative therapies such as androgens or hematopoietic cell gene correction may best be reserved for patients without donors or those who have risk factors for high mortality (eg, older age, poor organ function). Today, most FA patients with standard risk disease are cured of their BM failure by HCT even if an HLA-matched sibling donor is not available.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the nurses, nurse coordinators, nurse practitioners, physician assistants, social workers and physicians who cared for these patients and their families. The authors especially thank the patients with Fanconi anemia and their families who have entrusted us with their care to continually strive to improve upon our work.
This study was supported in part by a grant from the National Institutes of Health, National Cancer Institute (grant P01 CA65493), the Fanconi Anemia Research Fund, Children’s Cancer Research Fund, and Kidz1stFund.
Authorship
Contribution: M.L.M., T.E.D., B.R.B., K.E.D., and J.E.W. designed the research; all authors performed the research; T.E.D. performed the statistical analysis; all authors analyzed and interpreted the data; M.L.M. wrote the initial draft of the manuscript and edited the final draft; and all authors reviewed the draft manuscript and approved the final version for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Margaret L. MacMillan, Blood and Marrow Transplant Program, Department of Pediatrics, University of Minnesota, MMC 484, 420 Delaware St, SE, Minneapolis, MN 55455; e-mail: macmi002@umn.edu.