Abstract
The clinical challenge posed by p53-deficiency in hematological malignancies needs novel therapeutic strategies. ONC201, a first-in-class small molecule, was discovered as a p53-independent activator of apoptosis with a benign preclinical safety profile (Allen et al, Sci Transl Med 2013). Here we report that ONC201 exerts anti-tumor effects in hematological malignancies and leukemia stem cells (LSC) via the induction of ATF4 in the integrated stress response (ISR).
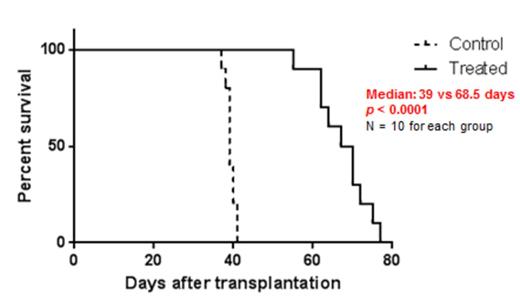
ONC201 induced p53-independent apoptosis in cell lines and primary patient samples from mantle cell lymphomas (MCL) and acute myeloid leukemias (AML), independent of genetic alterations that correlate with poor prognosis (e.g., TP53 mutation, FLT3-ITD, or complex karyotype). ONC201 also induced apoptosis in LSC, while sparing normal bone marrow progenitors, as measured in vitro and after transplantation. Specifically, we recovered unfractionated LSC-containing populations of AML cells (t(9;11)(p22; q23), CEBPA and ATM mutant) from secondarily-engrafted mice and cultured them in vitro for 48 hr in two groups, ONC201-treated (5 uM) or Control. For both groups, the same number of Trypan Blue-negative cells was then re-transplanted. The frequency of human CD45+ cells 4 weeks after transplantation was 38.09 ± 2.59 % of tibial BM cells in untreated mice and 0.10 ± 0.05% in treated mice (n = 3 for each, p < 0.01). The survival of the treated group was dramatically prolonged (Figure 1).
Gene Set Enrichment Analysis (GSEA) of gene expression profiling (GEP) data of Jeko-1 and Z-138 cells treated with ONC201 implicated up-regulated Endoplasmic Reticulum (ER) stress-related genes, such as targets of the ER stress-induced transcription factor CHOP (DDIT3; FDR q = 0.016), and ER component proteins (FDR q = 0.039). We confirmed the ONC201-induced increased mRNA levels of DDIT3, GADD34, DR5 and TRIB3, and increased protein levels of CHOP, ATF4 and IRE1-a in Jeko-1 cells. Knockdown of ATF4 and IRE1-a revealed that ATF4 is an essential protein for ONC201-induced apoptosis in most AML cells and MCL cells, while IRE1-a is only necessary for apoptosis in MCL cells. However, unlike other ER stress inducers, ONC201 did not cause phosphorylation of an eIF2a kinase PERK, a hallmark of classical ER stress. Importantly, ONC201 was also effective in lymphoma cells resistant to bortezomib, an ER stress inducer, supporting the notion that ONC201 uses a unique mechanism to trigger the ISR. In addition, ONC201 inhibited mTORC1 signaling, likely secondary to ATF4 activation via the induction of DDIT4, a negative regulator of mTORC1, which presumably increases the cytotoxicity of ONC201 by global translation inhibition.
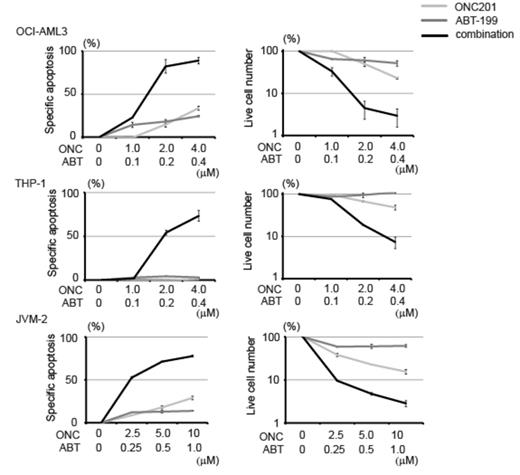
Investigating the type of apoptosis induced by ONC201, we examined protein levels of anti-apoptotic BCL-2 family members (BCL-2, BCL-XL and MCL-1) after ONC201 treatment. MCL-1 was reduced most notably after 12 hr. We then tested the effect of ONC201 on cells with overexpression or knockdown of BCL-2 family proteins. MCL-1 knockdown in OCI-AML3 cells increased their sensitivity to ONC201 only slightly, but ONC201 efficacy was dramatically reduced in BCL-2-overexpressing HL-60 cells, even more so than in BCL-XL-overexpressing HL-60 cells. Therefore, we investigated whether ONC201 sensitivity could be increased by ABT-199, a small-molecule BH3 mimetic that specifically inhibits BCL-2, and is known to be ineffective in cell lines with MCL-1 overexpression; accordingly, the combination of ONC201 and BCL-2 antagonist ABT-199 was highly synergistic (Figure 2).
In conclusion, ONC201 induces p53-independent apoptosis and abrogates LSC function by ATF4 induction, via the ISR. By suppressing MCL-1, ONC201 can also increase the effectiveness of the Bcl-2 inhibitor ABT-199. These findings suggest that ONC201 may provide promising novel therapeutic strategies for TP53 -wild type and TP53 -mutant hematological malignancies. Phase I/II clinical trials have been initiated at the MD Anderson Cancer Center in leukemias and lymphomas to determine safety, efficacy and further characterize mechanism of action.
Ishizawa:Karyopharm: Research Funding. Allen:Oncoceutics, Inc: Employment, Equity Ownership. Orlowski:Janssen Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Array BioPharma: Consultancy, Research Funding; Millennium Pharmaceuticals: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; BioTheryX, Inc.: Membership on an entity's Board of Directors or advisory committees; Acetylon: Membership on an entity's Board of Directors or advisory committees; Genentech: Consultancy; Forma Therapeutics: Consultancy; Onyx Pharmaceuticals: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Spectrum Pharmaceuticals: Research Funding. Wang:Celgene: Research Funding. Konopleva:Novartis: Research Funding; AbbVie: Research Funding; Stemline: Research Funding; Calithera: Research Funding; Threshold: Research Funding. Andreeff:Oncoceutics, Inc.: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.