Abstract
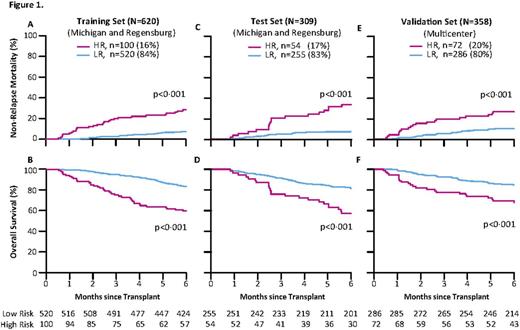
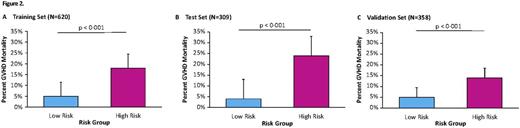
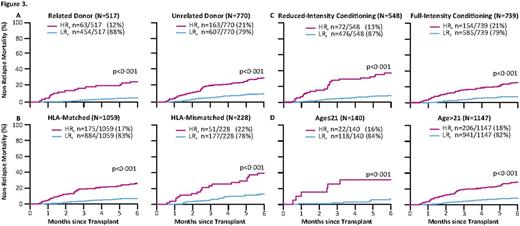
No laboratory test can predict non-relapse mortality (NRM) after hematopoietic cellular transplantation (HCT) prior to the onset graft-versus-host disease (GVHD). Recently, we have shown that a signature of three GVHD plasma biomarkers (TNFR1, ST2, and REG3α) can predict response to GVHD therapy and NRM at the onset of clinical GVHD (Levine, Lancet Haem, 2015). Our goal in the current study was to identify a blood biomarker signature that could predict lethal GVHD and six-month NRM well in advance of the onset of GVHD symptoms. Patient samples on day +7 after HCT were obtained from 1,287 patients from 11 HCT centers in the Mount Sinai Acute GVHD International Consortium (MAGIC). Samples from two large centers (n = 929) were combined and randomly assigned to a training set (n = 620) and test set (n = 309). 358 patients from nine others centers constituted an independent validation set. The overall cumulative incidences of 6-month NRM were 11%, 12%, and 13% for the training, test, and validation sets respectively. The incidence of lethal GVHD, defined as death without preceding relapse while under steroid treatment for acute GVHD, were 18%, 24%, and 14% in the same groups, respectively. The median day of GVHD onset was 28 days in the training set and 29 days in the test and validation sets. We measured four GVHD related biomarkers [ST2, REG3α, TNFR1, and IL2Rα] in all samples and used the training set alone to develop competing risks regression models that used all 13 possible combinations of one to four biomarkers to predict 6-month NRM. The best algorithm, which we rigorously confirmed through Monte Carlo cross-validation of 75 different combinations of training sets, included ST2 and REG3α. No combination of one, three, or four biomarkers was superior to the combination of these two biomarkers. The day 7 algorithm identified high risk (HR) and low risk (LR) groups with 6-month NRMs of 28% and 7%, respectively (p<0.001) (Fig 1A). The relapse rates did not differ between risk groups so that overall survival (OS) was 60% for HR and 84% for LR (p<0.001) (Fig 1B). When applied to the test set (Fig 1C/D), the algorithm identified 54/309 (17%) of the patients as HR with an NRM of 33% vs 7% for LR patients (p<0.001) and 6-month OS of 57% and 81% for HR and LR patients, respectively (p<0.001). In the independent validation set (Fig 1 E/F), the algorithm identified 72/358 (20%) of the patients as HR with an NRM of 26% vs 10% for LR patients (p<0.001) and OS of 68% and 85% for HR and LR patients, respectively (p<0.001). High risk patients were three times more likely to die from GVHD than LR patients in each cohort (p<0.001) (Fig 2). The GI tract is the GVHD target organ that is most resistant to treatment and represents a major cause of NRM, and we observed twice as much severe GI GVHD (stage 3 or 4) in HR patients as in LR patients (p<0.001, data not shown). The algorithm successfully separated HR and LR strata for 6 month NRM in several groups with differing risks for GVHD and NRM, including donor type, degree. of HLA-match, age group, and conditioning regimen intensity (Fig 3).
In conclusion, we have developed a blood biomarker algorithm that predicts the development of lethal GVHD seven days after HCT, which performed successfully in large multicenter validation sets. The GVH reaction is already in progress by day +7, even though clinical symptoms may not occur until days or weeks later. We speculate that the blood biomarker concentrations at this early time point reflect subclinical GI pathology, a notion that is reinforced by the fact that ST2 and REG3α, the two biomarkers in the algorithm, are closely associated with GI GVHD. The algorithm identified HR and LR strata in several patient groups with different overall risk for lethal GVHD (donor, HLA match, conditioning regimen intensity, age). This day +7 algorithm should prove useful in clinical BMT research by identifying patients at high risk for lethal GVHD who might benefit from aggressive preemptive treatment strategies.
Chen:Novartis: Research Funding; Incyte Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Jagasia:Therakos: Consultancy. Kitko:Therakos: Honoraria, Speakers Bureau. Kroeger:Novartis: Honoraria, Research Funding. Levine:Viracor: Patents & Royalties: GVHD biomarkers patent. Ferrara:Viracor: Patents & Royalties: GVHD biomarkers patent.
Author notes
Asterisk with author names denotes non-ASH members.