To the editor:
The course of aplastic anemia (AA) is often complicated by the development of clonal disorders such as paroxysmal nocturnal hemoglobinuria (PNH) and secondary myelodysplastic syndromes (sMDS).1-5 Identification of patients at risk for development of sMDS following AA, and distinguishing them from those with primary hypoplastic MDS (hypo-MDS) resembling AA, is important for the timely initiation of appropriate therapy. To determine potential discriminating features, we compared mutational disease evolution patterns among patients with AA, PNH, sMDS, hypo-MDS, and typical primary normo/hypercellular MDS (hyper-MDS).
Bone marrow and/or blood samples were collected from 258 AA and 59 PNH cases at Cleveland Clinic and University Hospital Basel (supplemental Tables 1 and 2, available on the Blood Web site). Among them, 35 patients whose initial AA or PNH progressed to sMDS were identified (Table 1; supplemental Tables 1, 2B, and 3). For comparison, we assembled a cohort of 853 patients with primary MDS (pMDS) that included 28 hypo-MDS and 825 hyper-MDS (supplemental Tables 1 and 2A; for details, see supplemental Materials and methods).6,7 We assessed copy number alterations by single nucleotide polymorphism (SNP) array karyotyping8,9 and somatic mutations by whole exome sequencing (supplemental Figure 1) and targeted deep sequencing (supplemental Table 4).
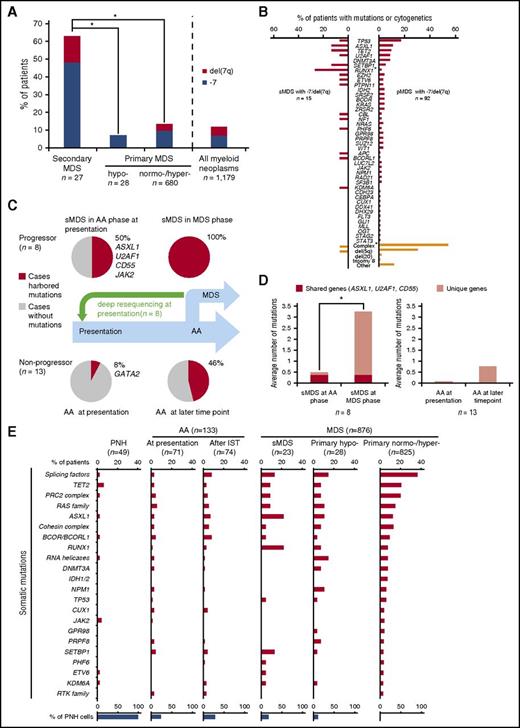
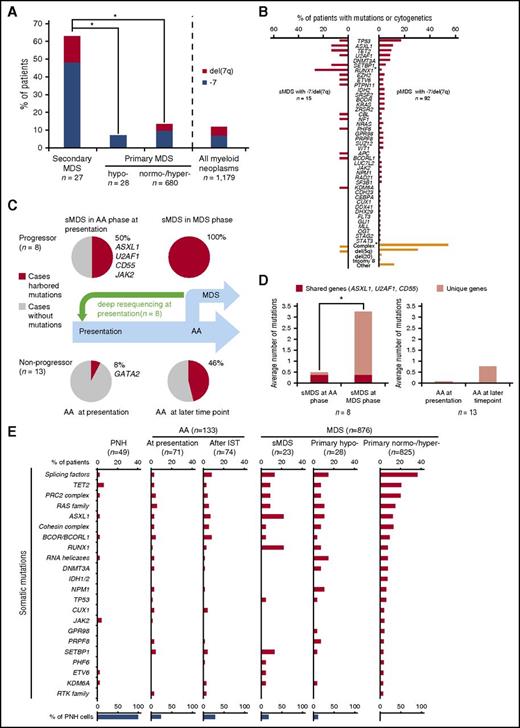
First, we analyzed all AA and MDS comparison groups serving as disease controls by targeted deep sequencing (supplemental Table 4). Somatic mutations were detected in 69/133 AA patients (32/71 at presentation vs 42/74 cases after IST; 12 cases were included in both cohorts). In contrast, acquired alterations were detected in 15/23 sMDS patients and in 657/853 pMDS patients (supplemental Figure 2A). As previously shown,5 most sMDS patients (63%) were characterized by −7/del(7q) evolution (Figure 1A; supplemental Table 3). By comparison, only 14% of pMDS patients had −7/del(7q), as assessed by both metaphase cytogenetics and SNP arrays. The average number of somatic mutations by targeted sequencing was 0.8, 1.0, 1.5, 1.5, and 2.0 in PNH, AA, sMDS, hypo-MDS, and hyper-MDS, respectively (supplemental Table 4; supplemental Figure 2B). In total, no mutations were found by targeted screening panels in 73% and 48% of PNH (except for PIGA mutations) and AA cases, respectively, whereas only 35% and 23% of sMDS and pMDS did not harbor detectable somatic mutations when assessed by the same method. In 8/15 sMDS and 66/92 pMDS patients with −7/del(7q), >1 somatic mutation was detected (Figure 1B). Comparison of survival between sMDS cases with and without mutations did not differ.
Genotypic and clinical features sMDS and pMDS including those with −7/del(7q). (A) Proportion of −7/del(7q) in sMDS (n = 27) compared with that in pMDS (hypo-MDS, n = 28; normo-/hyper-MDS, n = 680) (*P < .001). Overall, 14% of patients with myeloid neoplasms (n = 1179) showed −7/del(7q). (B) Mutational spectrum in −7/del(7q) patients with sMDS (n = 15) vs pMDS (n = 92) (*P < .01). (C-D) Paired whole exome sequencing or targeted deep sequencing was performed in sMDS, and any somatic mutations were identified in 8 cases. After driver mutations were identified, a custom targeted deep sequencing panel was designed and applied to the corresponding samples obtained at AA presentation. Mutations detected at both time points and fractions of patients in whom mutations were detected are shown. List of the genes affected is provided. Average numbers of mutations were shown in subsequent progressors and nonprogressors (*P = .005). (E) Individual bars represent fractions of cases with specific gene mutations among 49 PNH, 133 AA, and 876 MDS cases (supplemental Table 1; see also supplemental Materials and Methods). Supplemental Table 4 describes the multiamplicon sequencing panel. Significant differences in the distribution of mutations were shown in supplemental Table 5. Mutated genes were grouped according to functional relationships: splicing factors (SF3B1, SRSF2, U2AF1/2, and ZRSR2); RAS family (KRAS, NF1, NRAS, and PTPN11); PRC2 complex genes (EED, EZH2, and SUZ12); cohesin complex genes (RAD21, SMC3, and STAG2); RNA helicases (DDX41, DDX54, and DHX29); and RTK family (CSF1R, FLT3, and KIT). sMDS (post-AA MDS or post-PNH MDS); % of PNH cells defined as ratio of patients with PNH cells (>1%) detected by flow cytometry or with PIGA mutations identified by deep sequencing. There were 12 AA cases in both at presentation (before IST) and after IST cohort. AML, acute myeloid leukemia.
Genotypic and clinical features sMDS and pMDS including those with −7/del(7q). (A) Proportion of −7/del(7q) in sMDS (n = 27) compared with that in pMDS (hypo-MDS, n = 28; normo-/hyper-MDS, n = 680) (*P < .001). Overall, 14% of patients with myeloid neoplasms (n = 1179) showed −7/del(7q). (B) Mutational spectrum in −7/del(7q) patients with sMDS (n = 15) vs pMDS (n = 92) (*P < .01). (C-D) Paired whole exome sequencing or targeted deep sequencing was performed in sMDS, and any somatic mutations were identified in 8 cases. After driver mutations were identified, a custom targeted deep sequencing panel was designed and applied to the corresponding samples obtained at AA presentation. Mutations detected at both time points and fractions of patients in whom mutations were detected are shown. List of the genes affected is provided. Average numbers of mutations were shown in subsequent progressors and nonprogressors (*P = .005). (E) Individual bars represent fractions of cases with specific gene mutations among 49 PNH, 133 AA, and 876 MDS cases (supplemental Table 1; see also supplemental Materials and Methods). Supplemental Table 4 describes the multiamplicon sequencing panel. Significant differences in the distribution of mutations were shown in supplemental Table 5. Mutated genes were grouped according to functional relationships: splicing factors (SF3B1, SRSF2, U2AF1/2, and ZRSR2); RAS family (KRAS, NF1, NRAS, and PTPN11); PRC2 complex genes (EED, EZH2, and SUZ12); cohesin complex genes (RAD21, SMC3, and STAG2); RNA helicases (DDX41, DDX54, and DHX29); and RTK family (CSF1R, FLT3, and KIT). sMDS (post-AA MDS or post-PNH MDS); % of PNH cells defined as ratio of patients with PNH cells (>1%) detected by flow cytometry or with PIGA mutations identified by deep sequencing. There were 12 AA cases in both at presentation (before IST) and after IST cohort. AML, acute myeloid leukemia.
In AA, the spectrum of mutations on cross-sectional analyses differed from that of sMDS and pMDS (supplemental Figure 3; supplemental Table 5). ASXL1, RUNX1, splicing factors, and CBL mutations were significantly more common in sMDS compared with AA. Comparing sMDS with pMDS (either normo- or hypercellular), RUNX1 mutations were significantly more frequent in sMDS, whereas SF3B1 mutations were significantly less common. Interestingly, although DNMT3A mutations occurred in patients with AA (2/69 cases), they were absent in post-AA MDS (0/15 cases), suggesting that the mutagenic event did not initiate the MDS clonal cascade (supplemental Figure 3; supplemental Table 5). BCOR/BCORL1 mutations were also present in AA and expanded during the course of IST. However, the clonal burden was lower for BCOR/BCORL1 mutations than for other mutations when VAFs for specific mutations were compared in cases with multiple mutations. This suggests the secondary role of BCOR/BCORL1 mutations in the clonal hierarchy (supplemental Figure 3; supplemental Table 5).
Although −7/del(7q) was a characteristic feature of sMDS that evolved from AA (Figure 1A), post-AA sMDS with −7/del(7q) and pMDS with −7/del(7q) differed. TP53 mutations appeared to be more common in pMDS with −7/del(7q), yet RUNX1, ASXL1, TET2, and SETBP1 mutations appeared to be overrepresented in sMDS with −7/del(7q), but because of low numbers of event, the difference was significant only for RUNX1 (P = .003; Figure 1B).
Moreover, −7/del(7q) in pMDS was frequently associated with a complex karyotype (1/17 vs 50/92, P < .001) and del(5q) (1/17 vs 28/92, P = .04), whereas −7/del(7q) in sMDS tended to be the sole abnormality (14/16 cases; Figure 1B; supplemental Table 3).
Subsequently, we serially analyzed a cohort of 21 AA cases (8 patients who progressed to sMDS and 13 nonprogressors). At presentation, mutations were more frequently found in progressors than in nonprogressors (50% vs 8%, respectively; P = .048; Figure 1C), suggesting that certain clonal events seen in the MDS stage of the disease are indeed acquired early at presentation of AA and that some early hits may lead to subsequent clonal evolution. Similarly, the average number of mutations was higher in subsequent progressors than in nonprogressors (3.4 vs 0.7; P = .005; Figure 1D). In addition, mutated genes found already at AA presentation in subsequent MDS progressors included ASXL1, U2AF1, and JAK2 (Figure 1C,E). As indicated in supplemental Figures 4 and 5, the presence of the same mutation (in the same position in the same gene) at presentation and at evolution suggests a pathogenic role of this mutation and evidence of a clonal continuum.
In serial samples in AA without evolution, clones with GATA2, PHF6, RUNX1, SMC3, TET2, and BCORL1 mutations contracted during the course of AA, whereas ASXL1, CALR, CUX1, ETV6, EZH2, G3BP1, RIT1, U2AF1, and ZRSR2 expanded. In contrast, DNMT3A, BCOR, and CEBPA clones showed individually variable behavior with regard to clonal dynamics (supplemental Figure 4).
Clonal hierarchy was also assessed through a combination of allelic imbalance analyses and mutational burden (supplemental Materials and methods) in informative cases (Table 1; supplemental Figures 4 and 5) to determine whether mutations characterizing the MDS stage were already present at AA presentation or evolved during the course of disease. Analyses of clonal burden for mutations and −7/del(7q) revealed that deletions were the initial events in 3/5, whereas in 2/5 analyzed patients somatic mutations (CD55, TRIML1, TUSC3, ZNF208, RUNX1, PHF6, and SETBP1) preceded the acquisition of −7/del(7q) (supplemental Figures 4 and 5).
Somatic mutations may have clinical applications as diagnostic or prognostic markers. To assess the potential impact of somatic mutations on the outcomes of IST, we investigated a subset of AA patients (n = 37) who subsequently received IST. The presence of clonal somatic alterations (identified in 6/25 responders and 4/12 IST-refractory cases) did not predict efficacy of IST, consistent with the transient nature of most of these events. Within the serial cases (patients with AA at presentation), 12 cases subsequently developed sMDS (median time to progression, 3.3 years; range, 0.5-6.8). We also determined the clinical impact of MDS-driver mutations found in AA at presentation: AA patients who had any of the mutational hits found both at initial presentation and in subsequent MDS (n = 4) had a shorter median progression-free survival (2.0 years vs not reached, P < .001) and overall survival (2.6 years vs not reached, P = .02) when compared with cases without such somatic alterations (n = 67; supplemental Figure 6).
In sum, clonal somatic events can be detected in AA.6,10-12 Although most of these events, found typically in MDS, reflect clonal hematopoiesis and do not occur in or predict sMDS, certain founder mutations can be found at presentation in AA and have the potential to initiate progression to sMDS. These ancestral events could represent the first facilitating hit, leading to the acquisition of subsequent somatic lesions and the initiation of evolution to MDS. Other mutations indicate the clonality status of hematopoiesis and are not likely to lead to malignant disease.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The results published here were partly based on data generated by The Cancer Genome Atlas (TCGA) pilot project established by the National Cancer Institute and National Human Genome Research Institute. The authors are grateful to A. Wodnar-Filipowicz and A. Tichelli from the University Hospital in Basel, Switzerland, for providing patients’ samples. Information about TCGA and the investigators and institute for the TCGA research network can be found at http://cancergenome.nih.gov.
This work was supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (RO1HL118281, RO1HL123904, and RO1HL128425) (J.P.M.), and by the Edward P. Evans Foundation (J.P.M. and M.A.S.). Wenyi Shen was supported by a grant from the National Natural Science Foundation of China (81400079).
Contribution: E.N., H.M., and J.P.M. were responsible for overall design, data collection, analysis, interpretation, statistical analysis, manuscript preparation, and writing and completion of the manuscript; C.H., B.P., and R.Z.M. collected samples and analyzed data; Y.N., M.J.C., N.H., W.S., A.N., T.Y., B.P., T.K., M.A.S., and S.O., analyzed data and edited the manuscript; and all authors approved the final version of the manuscript and its submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jaroslaw P. Maciejewski, Cleveland Clinic, 9500 Euclid Ave/R40, Cleveland, OH 44195; e-mail: maciejj@ccf.org.