Key Points
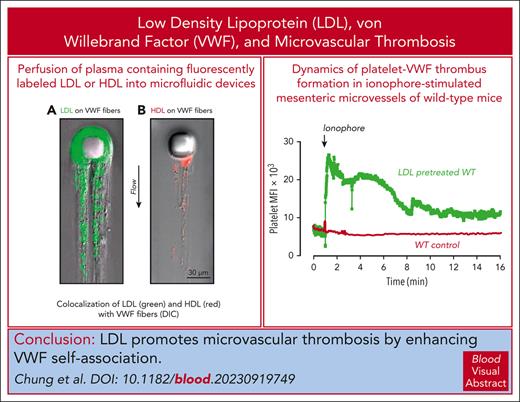
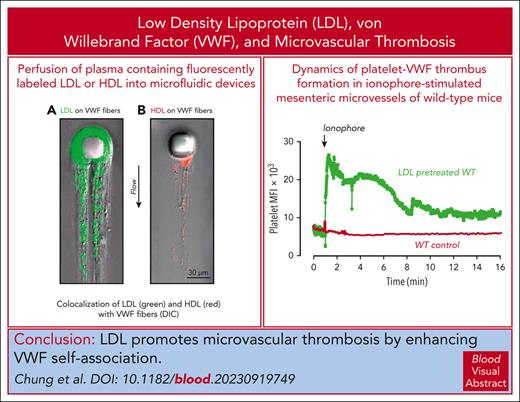
HDL attenuates and LDL enhances shear-induced VWF self-association in vitro and in vivo.
Elevated LDL increases formation of VWF-platelet thrombi in the myocardial microvasculature and other microvascular beds.
Abstract
von Willebrand factor (VWF) mediates primary hemostasis and thrombosis in response to hydrodynamic forces. We previously showed that high shear promoted self-association of VWF into hyperadhesive strands, which can be attenuated by high-density lipoprotein (HDL) and apolipoprotein A-I. In this study, we show that low-density lipoprotein (LDL) binds VWF under shear and enhances self-association. Vortexing VWF in tubes resulted in its loss from the solution and deposition onto tube surfaces, which was prevented by HDL. At a stabilizing HDL concentration of 1.2 mg/mL, increasing concentrations of LDL progressively increased VWF loss, the effect correlating with the LDL-to-HDL ratio and not the absolute concentration of the lipoproteins. Similarly, HDL diminished deposition of VWF in a post-in-channel microfluidic device, whereas LDL increased both the rate and extent of strand deposition, with both purified VWF and plasma. Hypercholesterolemic human plasma also displayed accelerated VWF accumulation in the microfluidic device. The initial rate of accumulation correlated linearly with the LDL-to-HDL ratio. In Adamts13−/− and Adamts13−/−LDLR−/− mice, high LDL levels enhanced VWF and platelet adhesion to the myocardial microvasculature, reducing cardiac perfusion, impairing systolic function, and producing early signs of cardiomyopathy. In wild-type mice, high plasma LDL concentrations also increased the size and persistence of VWF-platelet thrombi in ionophore-treated mesenteric microvessels, exceeding the accumulation seen in similarly treated ADAMTS13-deficient mice that did not receive LDL infusion. We propose that targeting the interaction of VWF with itself and with LDL may improve the course of thrombotic microangiopathies, atherosclerosis, and other disorders with defective microvascular circulation.
Introduction
von Willebrand factor (VWF) is a large multimeric plasma glycoprotein that mediates platelet adhesion to sites of vessel injury.1 VWF is synthesized by endothelial cells and megakaryocytes and assembled into a series of disulfide-linked multimers that can reach enormous sizes.2 The bulk of VWF is stored in the Weibel-Palade bodies of endothelial cells and α granules of platelets3 and secreted upon cell activation. Newly secreted VWF contains ultralarge multimers that are prothrombotic but are usually converted to smaller, less adhesive multimers by the plasma metalloprotease ADAMTS13.4 A portion of the secreted VWF remains attached to the endothelial surface and is stretched by the flowing blood, unfolding domains, and exposing sites for platelet binding, ADAMTS13 cleavage, or associating with other surface-attached or circulating VWF multimers.5 VWF self-association can produce longer, thicker fibers that efficiently capture platelets and is favored in regions of elevated shear stress, flow acceleration, or turbulence.6-9 The extent of VWF self-association is also affected by a competition between ADAMTS13-mediated cleavage and the binding of VWF multimers to each other.10 When the rate of VWF self-association exceeds cleavage, VWF-platelet thrombi accumulate and obstruct blood flow, causing tissue ischemia and infarction. This mechanism contributes to thrombosis in thrombotic thrombocytopenic purpura11 and other thrombotic microangiopathies,12 arterial and venous thrombosis,13-15 malaria,16 sepsis,17 and sickle cell disease18 and promotes progression of atherosclerosis.19-24 These findings suggest that interference with VWF self-association can be exploited to alleviate a wide variety of thrombotic complications associated with cardiovascular and inflammatory diseases.
We previously observed that high-density lipoprotein (HDL) and its major apolipoprotein A-I (ApoA-I) attenuated VWF self-association.5 Platelet binding to VWF fibers was reduced in proportion to the reduction in the fibers. In a mouse model of thrombotic microangiopathy (TMA), HDL reduced the consumptive thrombocytopenia induced by high doses of VWF. An inverse correlation between HDL and hyperadhesive VWF levels has also been observed in patients with thrombotic thrombocytopenic purpura (TTP) and sepsis.5 Thus, VWF self-association is regulated not only by hydrodynamic forces and ADAMTS13 but also by other plasma components. In this study, we assessed the role of LDL on VWF self-association under shear. Unlike HDL, LDL markedly accelerated and magnified the self-association process in vitro and in a VWF-dependent manner, impaired microvascular blood flow in vivo. The interaction between elevated LDL and VWF may shed light on the mechanism for the observation that 1 meal rich in saturated fat induced blood sludging and blood-cell aggregation in the microvasculature of hamster25 and for the finding that acute hypercholesterolemia in experimental animals increased myocardial infarct size and markedly impaired microvascular reperfusion.26
Methods
Materials
Human VWF was purified from cryoprecipitate as described.27 The multimer profile was determined by sodium dodecyl sulfate–agarose gel electrophoresis and western blotting.28 Purified VWF (5 and 10 μg) supported ristocetin-induced platelet agglutination comparable with that of normal platelet-poor plasma (not shown). Expression of biotinylated recombinant VWF (Bio-VWF) was described in the supplemental Data, available on the Blood website, of our previous publication.5 VWF concentration was measured using enzyme-linked immunosorbent assay (ELISA) with polyclonal antibodies (DAKO, North America Inc). P3A11 is an inhibitory monoclonal antibody to human ADAMTS13 produced at the Bloodworks Research Institute.
Isolation of HDL and LDL
HDL and LDL were isolated from pooled citrated human plasma by sequential potassium bromide density gradient ultracentrifugation, as described.29 Purity was assessed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and protein staining. The concentrations of HDL and LDL, expressed as mg/mL of particles, were calculated from the ApoA-I, ApoB100, total protein, and cholesterol content as described in supplemental Data.
Shear-induced VWF self-association in tubes
Bio-VWF or diluted citrated plasma in polypropylene microfuge tubes was vortexed on a rotary mixer (MixMate, Eppendorf) at 3000 rpm for 1.5 or 3 hours at 21°C in the presence of various concentrations of HDL and LDL. Vortexing caused VWF to deposit on tube surfaces, resulting in its loss from solution.5 VWF remaining in solution was quantified using ELISA.
Shear-induced VWF self-association in microfluidic devices
Purified VWF or plasma was mixed with various concentrations of purified HDL and LDL and perfused through microfluidic devices adapted from the design of Herbig and Diamond.30 High shear stress and flow acceleration in this device promoted adhesion of VWF to the post and growth of VWF fibers through self-association. The VWF fibers were visualized either with fluorescein isothiocyanate (FITC)–labeled anti-VWF antibodies (Abcam) or using differential interference contrast (DIC) videography with an Olympus IX81 microscope. Image analysis and quantification of VWF accumulation are described in supplemental Data.
Patients with hypercholesterolemia
EDTA-anticoagulated plasma samples were selected from the biorepository of the Center for Preventive Cardiology at Oregon Health and Science University (IRB #17329), which enrolls patients with genetic dyslipidemias, extreme lipid phenotypes, or atherosclerotic cardiovascular disease. Plasma was selected from participants matched for age, gender, and HDL-C levels but with LDL-C >150 mg/dL.
In vivo assessment of microvascular VWF, platelet adhesion, and perfusion
The Animal Care and Use committee of Oregon Health and Science University approved the procedures used in the studies presented in this section. We used a murine model to study the role of LDL in TMA. Both Adamts13−/− mice (17-19 weeks old) and Adamts13−/−LDLR−/− mice (23-25 weeks old) with a C57BL/6 background were used. Adamts13−/−LDLR−/− mice were put on a high-fat diet for 2 weeks before the experiments. A VWF challenge (5 mg/kg, IV)5 or control saline was administered 1 hour before study. Half of the Adamts13−/− mice received purified human LDL (300 mg/kg, intraperitoneally [IP]) 2 hours before the study. Myocardial microvascular perfusion was assessed noninvasively using quantitative contrast echocardiography, which provided parametric information on myocardial microvascular blood volume (MBV), microvascular flux rate (β), and microvascular blood flow (calculated as the product of MBV and β), as previously reported.31 Endothelium-associated VWF or GPIbα on immobilized platelets was detected using ultrasound molecular imaging with targeted microbubbles. Imaging was performed 8 minutes after IV injection of microbubbles bearing either a biotinylated N-terminal 300-amino acid fragment of GPIbα or a dimeric murine VWF A1 fragment (amino acids 445-716) to detect VWF and platelets, respectively.32 Nontargeted microbubbles served as control.
Ionophore-provoked microvascular thrombosis model
The Animal Care and Use committee of the University of Michigan, the former institution of Reheman Adili, approved the procedures used in the following studies. Wild-type (WT) C57BL/6 mice (3-4 week old) were prepared as described.28,33 Preparation of animals, ionophore provocation, imaging and data analysis are described in supplemental Data. LDL (IV, 100 mg/kg) was injected into recipient mice via tail veins 10 minutes before ionophore provocation.
Quantification of VWF cleavage by ADAMTS13
The extent of VWF cleavage, expressed as percent VWF cleaved to total VWF, was determined by the ratio of signature tryptic peptides derived from cleaved and uncleaved VWF, using mass spectrometry as described in supplemental Data. Synthetic heavy isotope–labeled reference peptides were used for quantification in these studies.
Statistical analysis
Data were compared using an unpaired, 2-tailed Student t test. An α of 0.05 was used for significance. All statistical analyses were performed with GraphPad Prism 8.1.1 (GraphPad Software, San Diego, CA).
Results
LDL enhances VWF self-association in tube-vortex studies
When a solution of Bio-VWF was sheared in a microfuge tube on a rotary vortexer, the Bio-VWF adsorbed and extensively self-associated on the tube surface; only small amounts remained in solution.5 As observed previously, HDL attenuated VWF surface adsorption and self-association in concentration-dependent manner (Figure 1A). The addition of various amounts of LDL to a stabilizing concentration of HDL to increase the LDL-to-HDL ratio progressively enhanced VWF surface adsorption and self-association (Figure 1B). Doubling both LDL and HDL levels but maintaining the LDL-to-HDL ratio enhanced surface adsorption and self-association to similar extents (Figure 1B). Thus, LDL and HDL have opposing effects on VWF self-association under shear, with HDL attenuating VWF self-association and LDL enhancing it. These observations also suggest that it is the ratio of LDL to HDL and not the concentration of lipoprotein particles that is important in regulating VWF self-association. The addition of HDL to plasma with an endogenous LDL-to-HDL ratio of 1.43 (equivalent LDL-C–to–HDL-C ratio, 3.2) to lower the ratio to 0.45 (LDH-C–to–HDL-C ratio, 1.0) stabilized VWF in solution, whereas the addition of LDL (new ratio, 4.29; equivalent to the LDL-C–to–HDL-C ratio, 9.7) led to greater shear-induced VWF loss (Figure 1C).
VWF self-association at various HDL and LDL levels. Bio-VWF (5 μg/mL) in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4 was vortexed for 90 minutes at room temperature in the presence of increasing concentrations of HDL (A) or in the presence of either 1.2 or 2.4 mg/mL of HDL and increasing concentrations of LDL (B). The Bio-VWF remaining in solution was measured using ELISA. Bio-VWF in tubes not vortexed served as control (100%) (n = 3 in [A], n = 4 in [B]). (C) Citrated pooled human plasma (50%) in 10 mM EDTA was sheared by vortexing for 3 hours. VWF remaining in solution was measured using ELISA and expressed as a percentage of the VWF in parallel samples not exposed to shear stress (n = 3). The ratios of LDL to HDL are shown in both cholesterol ratios (LDL-C to HDL-C) and total particle weight ratios (LDL to HDL).
VWF self-association at various HDL and LDL levels. Bio-VWF (5 μg/mL) in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4 was vortexed for 90 minutes at room temperature in the presence of increasing concentrations of HDL (A) or in the presence of either 1.2 or 2.4 mg/mL of HDL and increasing concentrations of LDL (B). The Bio-VWF remaining in solution was measured using ELISA. Bio-VWF in tubes not vortexed served as control (100%) (n = 3 in [A], n = 4 in [B]). (C) Citrated pooled human plasma (50%) in 10 mM EDTA was sheared by vortexing for 3 hours. VWF remaining in solution was measured using ELISA and expressed as a percentage of the VWF in parallel samples not exposed to shear stress (n = 3). The ratios of LDL to HDL are shown in both cholesterol ratios (LDL-C to HDL-C) and total particle weight ratios (LDL to HDL).
High LDL-to-HDL ratios enhance VWF self-association in microfluidic devices
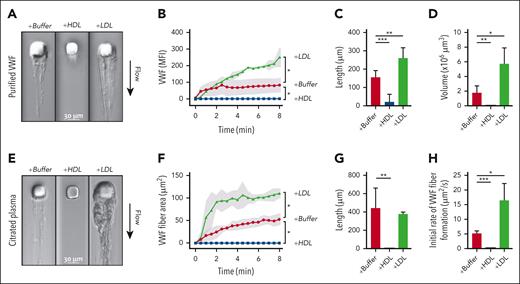
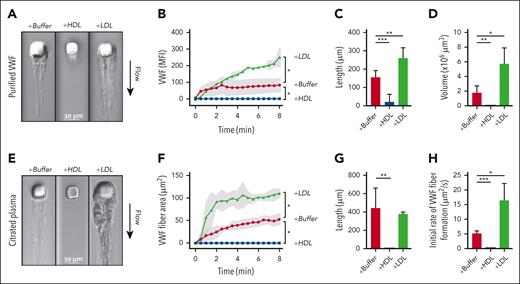
We studied VWF self-association using both purified components and citrated plasma in a microfluidic device, in which fluid perfused through a microchannel with a micropost created regions of high wall-shear stress and flow acceleration, which induce VWF attachment to the micropost and self-association into fibers around the micropost. With perfused purified VWF and a low concentration of FITC-labeled VWF antibodies, fluorescent VWF fibers accumulated on the micropost and extended downstream as sheets (Figure 2A). Progressive fiber accumulation was quantified based on the mean fluorescence intensity per area in the time-lapse images (Figure 2B; supplemental Video 1). In addition, fiber length (Figure 2C) and volume were determined at the end of the 16-minute perfusion (Figure 2D; supplemental Video 2). Through each of these measures, HDL attenuated and LDL enhanced VWF self-association under shear.
Effects of HDL and LDL on VWF self-association in microfluidic devices. (A-D) Solutions containing purified VWF (7.5 μg/mL) in phosphate-buffered saline, fluorescein isothiocyanate (FITC)–conjugated anti-VWF antibody, and HDL (2.4 mg/mL) or LDL (2.2 mg/mL) were perfused through microfluidic devices at an upstream wall-shear rate of 9300 s−1. VWF fibers formed around the micropost were monitored for both DIC and FITC intensities. Data were from 3 to 6 experiments. (A) DIC images of VWF fibers formed after 8 minutes of perfusion. (B) VWF fibers, proportional to the mean fluorescence intensity of the bound FITC-conjugated anti-VWF antibody, were quantified over time. Shading represents standard deviations (SDs). The length and volume of VWF fibers at the end of perfusion were quantified in (C) and (D), respectively. (E-H) Citrated plasma from healthy donors, with phosphate-buffered saline, HDL (1 mg/mL), or LDL (1 mg/mL) were perfused through microfluidic devices. VWF fibers formed around the micropost were monitored using DIC intensity. Data were from 3 to 7 experiments. (E) DIC images of VWF fiber formation after 8 minutes of perfusion. (F) VWF fiber areas were quantified over time. Shading represents SDs. (G) The lengths of VWF fibers at the end of perfusion were quantified. (H) The initial rates of VWF fiber formation were calculated. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 by t test.
Effects of HDL and LDL on VWF self-association in microfluidic devices. (A-D) Solutions containing purified VWF (7.5 μg/mL) in phosphate-buffered saline, fluorescein isothiocyanate (FITC)–conjugated anti-VWF antibody, and HDL (2.4 mg/mL) or LDL (2.2 mg/mL) were perfused through microfluidic devices at an upstream wall-shear rate of 9300 s−1. VWF fibers formed around the micropost were monitored for both DIC and FITC intensities. Data were from 3 to 6 experiments. (A) DIC images of VWF fibers formed after 8 minutes of perfusion. (B) VWF fibers, proportional to the mean fluorescence intensity of the bound FITC-conjugated anti-VWF antibody, were quantified over time. Shading represents standard deviations (SDs). The length and volume of VWF fibers at the end of perfusion were quantified in (C) and (D), respectively. (E-H) Citrated plasma from healthy donors, with phosphate-buffered saline, HDL (1 mg/mL), or LDL (1 mg/mL) were perfused through microfluidic devices. VWF fibers formed around the micropost were monitored using DIC intensity. Data were from 3 to 7 experiments. (E) DIC images of VWF fiber formation after 8 minutes of perfusion. (F) VWF fiber areas were quantified over time. Shading represents SDs. (G) The lengths of VWF fibers at the end of perfusion were quantified. (H) The initial rates of VWF fiber formation were calculated. ∗P < .05; ∗∗P < .01; and ∗∗∗P < .001 by t test.
Besides purified VWF, we also perfused citrated plasma and imaged the accumulation using DIC. VWF from plasma with an endogenous LDL-to-HDL ratio of 0.9 (LDH-C–to–HDL-C ratio, 2.0) progressively accumulated as fibers on the micropost (Figure 2E-H). Changing the endogenous lipoprotein ratio by adding LDL or HDL to the plasma to change the LDL-to-HDL ratios to 1.4 (LDH-C–to–HDL-C ratio, 3.2) or 0.6 (LDH-C–to–HDL-C ratio, 1.4), respectively, also enhanced and attenuated the accumulation of VWF fibers (Figure 2E-G; supplemental Video 3). These studies recapitulated the findings with purified components and showed that the effects of HDL and LDL on VWF self-association were readily evident in the complex milieu of plasma. LDL-enhanced fiber accumulation was rapid during the initial 2 minutes of perfusion, followed by a slower and steady rate (Figure 2F,H), which may be caused by a combination of increased shear forces and the proteolytic activity of the endogenous ADAMTS13 in plasma.
Binding of LDL to VWF under shear
When we added trace amounts of AF 488-labeled LDL to the plasma before perfusion, we observed colocalization of the LDL (fluorescent signal) with VWF fibers (DIC intensity) (supplemental Figure 1A; supplemental Video 4A), suggesting that LDL directly complexes with VWF to induce its self-association and deposition in the device.
When trace amounts of AF 647–labeled HDL was added to the perfused plasma, fewer VWF fibers formed, accompanied by scant decoration of the fibers with HDL (supplemental Figure 1B; supplemental Video 4B), consistent with the interpretation that HDL capped the self-association sites on the fibers and limited their elongation and thickening.
VWF self-association in the plasma from individuals with hypercholesterolemia
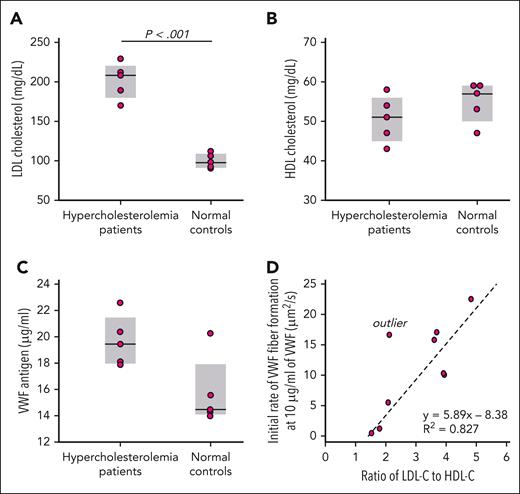
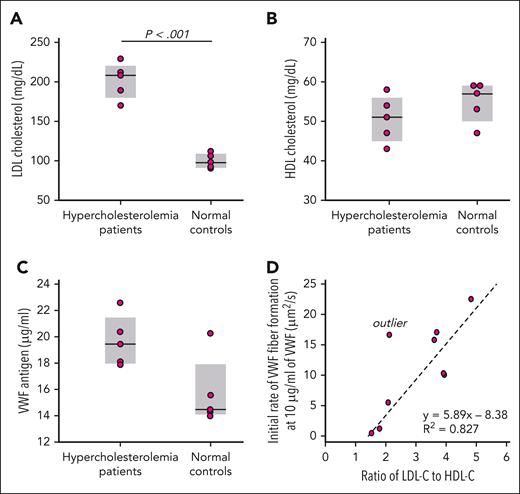
We studied the EDTA-anticoagulated plasma from 5 patients with hypercholesterolemia and 5 matched control participants (Figure 3A). The HDL levels were not significantly different between the 2 groups (Figure 3B). We measured the VWF levels in each sample (Figure 3C) and diluted each to 10 μg/mL VWF before perfusion. One control sample was hemolyzed and contained debris that blocked the microfluidic device and could not be studied. The rate of VWF fiber accumulation on the microposts during the initial 150 seconds correlated strongly with the LDL-C–to–HDL-C ratio (Figure 3D).
VWF fiber formation in plasma from patients with hypercholesterolemia. LDL cholesterol (A), HDL cholesterol (B), and VWF antigen (C) were measured in the EDTA plasma from patients with hypercholesterolemia (n = 5) and healthy controls (n = 5). (D) The plasma from the patients with hypercholesterolemia and healthy controls were diluted with saline to the same VWF concentration (10 μg/mL) before being perfused through microfluidic devices. VWF fiber formation was monitored in DIC channel. The initial rates of VWF fiber formation were calculated over the first 150 seconds of perfusions. The outlier was excluded from statistical analysis. Plasma of patients with hypercholesterolemia: n = 5; healthy controls: n = 4.
VWF fiber formation in plasma from patients with hypercholesterolemia. LDL cholesterol (A), HDL cholesterol (B), and VWF antigen (C) were measured in the EDTA plasma from patients with hypercholesterolemia (n = 5) and healthy controls (n = 5). (D) The plasma from the patients with hypercholesterolemia and healthy controls were diluted with saline to the same VWF concentration (10 μg/mL) before being perfused through microfluidic devices. VWF fiber formation was monitored in DIC channel. The initial rates of VWF fiber formation were calculated over the first 150 seconds of perfusions. The outlier was excluded from statistical analysis. Plasma of patients with hypercholesterolemia: n = 5; healthy controls: n = 4.
LDL and VWF in the mouse myocardial microcirculation
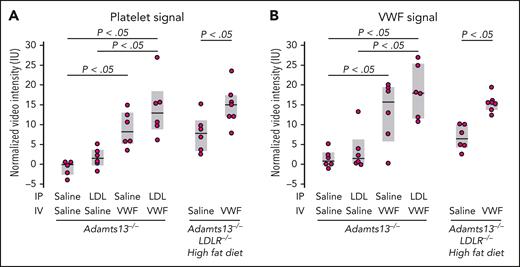
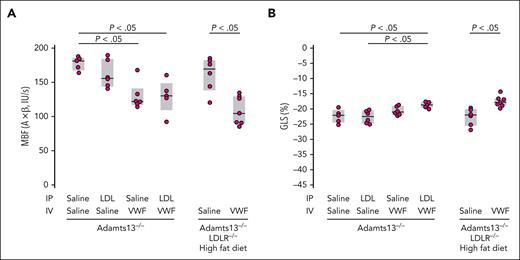
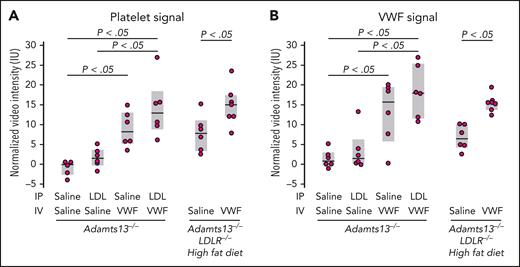
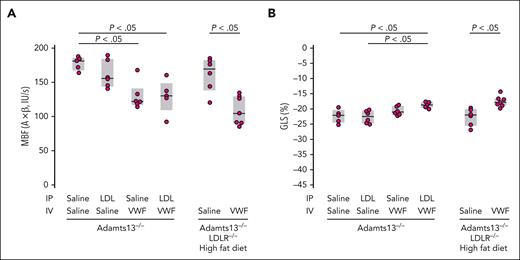
We hypothesized that enhanced VWF self-association in vivo would potentiate TMA. We, therefore, evaluated the effect of LDL in a mouse model of TMA based on ADAMTS13 deficiency and VWF challenge.34 We attempted to increase the LDL level in mice in 2 ways: by administering exogenous LDL IP in Adamts13−/− mice and by putting mice with combined ADAMTS13 and the LDL receptor (LDLR) deficiency on a high-fat diet. We evaluated the myocardial microcirculation by both molecular and perfusion imaging. The binding of VWF and platelets to the myocardial microcirculation was significantly increased in Adamts13−/− mice challenged with VWF (lane 3, Figure 4A-B). When this mouse strain was challenged with both VWF (administered IV) and LDL (administered IP), there was a modest increase in platelet and VWF binding (lane 4, Figure 4A-B). The modest increase could result from the already high VWF binding in the Adamts13−/− background and an inadequate rise in the plasma LDL level after IP injection. In parallel studies, Adamts13−/− mice injected IP with the same dose of LDL demonstrated only modest rises in circulating LDL-C levels (∼17 mg/dL; not shown), far below levels observed in mice with hyperlipidemia. We, therefore, used mice with combined deficiencies of ADAMTS13 and LDLR made hypercholesterolemic through a high-fat diet.32 After 2 weeks of fat feeding, circulating LDL-C levels in the mice were indeed high (LDL-C, ∼326 ± 122 mg/dL; supplemental Figure 2A) at the time of study. Even without the VWF challenge, there were significant increases in platelet and VWF signals on the microvascular endothelium (lane 5, Figure 4A-B), which was further enhanced by VWF challenge (lane 6, Figure 4A-B). This was accompanied by reduced myocardial blood flow (Figure 5A), which was largely secondary to reduced MBV rather than reduced β, implicating myocardial microvascular obstruction (supplemental Figure 3). These changes were evident in Adamts13−/− mice and more pronounced in double-knockout mice challenged with VWF. Furthermore, myocardial global longitudinal strain was also significantly decreased in both groups of mice (Figure 5B). Left ventricular ejection fraction stroke volume, and global circumferential strain were not significantly changed (supplemental Figure 4). Thus, hypercholesterolemia impaired left ventricular systolic function by inducing myocardial microangiopathy, a phenomenon exacerbated by the absence of ADAMTS13.
Contrast-enhanced ultrasound molecular imaging in Adamts13−/− and Adamts13−/−LDLR−/− mice.Adamts13−/−LDLR−/−mice (aged 23-25 weeks) were put on high fat diet for 2 weeks before imaging, with microbubbles functionalized to detect platelet (A) and VWF (B) signals on the myocardial microcirculation. A challenge with LDL (300 μg/g body weight; IP) was administered 2 hours before imaging in Adamts13−/− mice (aged 17-19 weeks). A challenge with VWF (5 μg/g body weight; IV) was administered 1 hour before imaging. P values were by t test.
Contrast-enhanced ultrasound molecular imaging in Adamts13−/− and Adamts13−/−LDLR−/− mice.Adamts13−/−LDLR−/−mice (aged 23-25 weeks) were put on high fat diet for 2 weeks before imaging, with microbubbles functionalized to detect platelet (A) and VWF (B) signals on the myocardial microcirculation. A challenge with LDL (300 μg/g body weight; IP) was administered 2 hours before imaging in Adamts13−/− mice (aged 17-19 weeks). A challenge with VWF (5 μg/g body weight; IV) was administered 1 hour before imaging. P values were by t test.
Examination of myocardial microvascular perfusion and function of the experimental mice in Figure 4 using echocardiography. Elevated levels of VWF and LDL decrease myocardial blood flow (MBF) (A) and global longitudinal strain (GLS) (B). (A) MBF, MBV, and β. (B) GLS, expressed as the percent change in heart muscle in the ventricle-apex axis at end-diastole. P values were by t test.
Examination of myocardial microvascular perfusion and function of the experimental mice in Figure 4 using echocardiography. Elevated levels of VWF and LDL decrease myocardial blood flow (MBF) (A) and global longitudinal strain (GLS) (B). (A) MBF, MBV, and β. (B) GLS, expressed as the percent change in heart muscle in the ventricle-apex axis at end-diastole. P values were by t test.
LDL enhances ionophore-provoked mesenteric thrombosis
We, next, examined the impact of high LDL levels on microvascular function in WT C57BL/6 mice by intravital microscopy of mesenteric venules. We have shown, previously, that in WT mice, VWF-platelet thrombi form upon ionophore stimulation, but the thrombotic response is very mild and brief compared to the response in ADAMTS13-deficient mice.28 This mild response suggested that the effect of LDL on the thrombotic response could be easily discerned in WT mice.
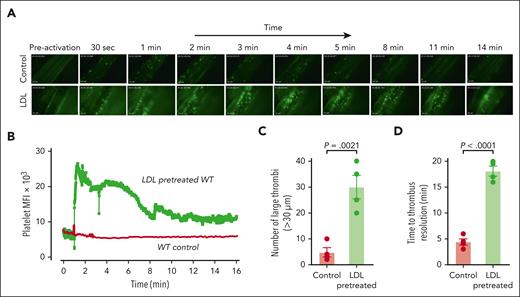
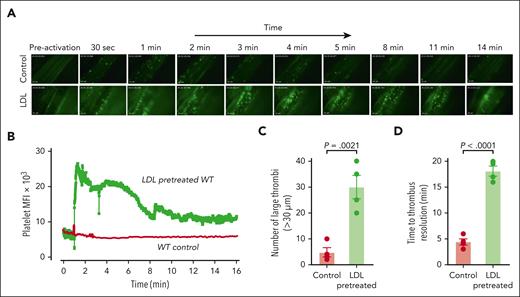
Application of ionophore to the mesenteric venules of WT mice led to immediate platelet adhesion as single platelets or small clusters (supplemental Video 5) with very few large thrombi (Figure 6A-C). Time to thrombus resolution and return to baseline platelet binding was less than 5 minutes (Figure 6D). In mice injected with LDL, the thrombi were much more numerous and larger (Figure 6A-C; supplemental Video 6), and the thrombotic response persisted longer than 16 minutes before platelet signals returned to baseline (Figure 6B,D). Endogenous ADAMTS13 activity was unable to prevent LDL-enhanced thrombosis.
LDL enhances microvascular thrombosis induced by calcium ionophore in WT C57BL/6 mice. WT mice (aged 3-4 weeks) were injected with platelets that were isolated from WT donors and fluorescently labeled with calcein AM ex vivo. Their mesenteric vessels were exposed and topically treated with calcium ionophore to induce VWF secretion. The dynamics of platelet VWF thrombus formation and resolution in mesenteric vessels were monitored and quantified. For the LDL-pretreated group, mice received 100 mg/kg of purified human LDL through tail-vein injection 10 minutes before calcium ionophore treatment. (A) Sequential images taken at the indicated times after the application of calcium ionophore. Images taken at similar times from the control and LDL-pretreated WT mice are aligned. Original magnification ×200. (B) Mean fluorescence intensity (MFI) of platelet thrombi was quantified for each frame of the recorded video and plotted against time. (C) Platelet thrombi >30 μm in diameter were quantified. (D) Time to thrombus resolution was quantified. Upon calcium ionophore stimulation, platelets immediately began to accumulate on the vessel wall. Adhesion was monitored, and the time required for the fluorescence value to return to baseline was measured. LDL treatment significantly prolonged the time required for platelet adhesion to return to baseline. The data were analyzed using Student t test; the P values are indicated.
LDL enhances microvascular thrombosis induced by calcium ionophore in WT C57BL/6 mice. WT mice (aged 3-4 weeks) were injected with platelets that were isolated from WT donors and fluorescently labeled with calcein AM ex vivo. Their mesenteric vessels were exposed and topically treated with calcium ionophore to induce VWF secretion. The dynamics of platelet VWF thrombus formation and resolution in mesenteric vessels were monitored and quantified. For the LDL-pretreated group, mice received 100 mg/kg of purified human LDL through tail-vein injection 10 minutes before calcium ionophore treatment. (A) Sequential images taken at the indicated times after the application of calcium ionophore. Images taken at similar times from the control and LDL-pretreated WT mice are aligned. Original magnification ×200. (B) Mean fluorescence intensity (MFI) of platelet thrombi was quantified for each frame of the recorded video and plotted against time. (C) Platelet thrombi >30 μm in diameter were quantified. (D) Time to thrombus resolution was quantified. Upon calcium ionophore stimulation, platelets immediately began to accumulate on the vessel wall. Adhesion was monitored, and the time required for the fluorescence value to return to baseline was measured. LDL treatment significantly prolonged the time required for platelet adhesion to return to baseline. The data were analyzed using Student t test; the P values are indicated.
Effect of ADAMTS13 on VWF self-association
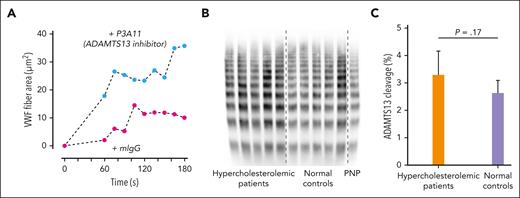
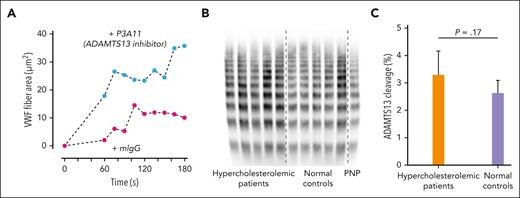
Although the binding of HDL to VWF under shear slightly reduced the cleavage by ADAMTS13 in tube-vortex experiments (not shown), whether LDL affects cleavage is not clear and cannot be precisely determined in vitro because of rapid VWF surface adsorption and incomplete elution from the surface of the adsorbed VWF using detergents. Alternatively, we evaluated the role of the endogenous ADAMTS13 on VWF self-association in microfluidic perfusion studies by inhibiting ADAMTS13 with the monoclonal antibody P3A11. This antibody, but not a nonspecific isotype-matched control antibody, significantly augmented the rate and extent of VWF fiber accumulation in the microfluidic device (Figure 7A), as did the inhibition of ADAMTS13 by EDTA (not shown).
Enhanced VWF fiber formation in the presence of an ADAMTS13 inhibitor and no effect of high LDL in patients with hypercholesterolemia on VWF cleavage by ADAMTS13. (A) Citrated plasma was incubated with inhibitory monoclonal antibody P3A11 (100 μg/mL) to ADAMTS13 or isotype control mouse antibody at room temperature for 1 hour before perfusion through microfluidic devices. VWF fiber formation was monitored and quantified over time. (B) VWF multimers in patients with hypercholesterolemia were similar to those of healthy controls. VWF multimers were analyzed using 1.7% agarose gel and immunoblotting. (C) VWF cleavages in vivo by ADAMTS13 measured by mass spectrometry in patients with hypercholesterolemia were similar to those in healthy controls. The data were analyzed using Student t test, and the P values are indicated. PNP, pooled normal plasma.
Enhanced VWF fiber formation in the presence of an ADAMTS13 inhibitor and no effect of high LDL in patients with hypercholesterolemia on VWF cleavage by ADAMTS13. (A) Citrated plasma was incubated with inhibitory monoclonal antibody P3A11 (100 μg/mL) to ADAMTS13 or isotype control mouse antibody at room temperature for 1 hour before perfusion through microfluidic devices. VWF fiber formation was monitored and quantified over time. (B) VWF multimers in patients with hypercholesterolemia were similar to those of healthy controls. VWF multimers were analyzed using 1.7% agarose gel and immunoblotting. (C) VWF cleavages in vivo by ADAMTS13 measured by mass spectrometry in patients with hypercholesterolemia were similar to those in healthy controls. The data were analyzed using Student t test, and the P values are indicated. PNP, pooled normal plasma.
Effect of LDL on VWF cleavage
We examined VWF cleavage by ADAMTS13 in the plasma samples from patients with hypercholesterolemia using nanoLC-MS/MS and VWF multimer profile. There were no differences in the percent of VWF cleaved by ADAMTS13 and the VWF multimer distribution between healthy individuals and patients with hypercholesterolemia (Figure 7B-C). Both results indicate that LDL does not significantly affect the extent of VWF cleavage by ADAMTS13 in vivo.
Discussion
In this study, we report that VWF self-association into hyperadhesive fibers is regulated by the ratio of LDL to HDL in the plasma. High LDL-to-HDL ratios, as those that occur in familial or acquired hypercholesterolemia, enhance VWF self-association, accompanied by the incorporation of LDL into the VWF fibers. In contrast, low LDL-to-HDL ratios attenuate self-association. At a high LDL-to-HDL ratio, VWF self-association can overwhelm the capacity of endogenous ADAMTS13 to cleave and clear the fibers.
Regulation of VWF self-association and, therefore, platelet adhesion by lipoproteins has important clinical implications. In the long term, the counterbalancing effects of HDL and LDL on VWF self-association would contribute to the genesis and progression of atherosclerosis, mainly a disease of large arteries. Indeed, important roles for VWF and ADAMTS13 in the initiation and progression of atherosclerosis have been reported.20,35-38 Direct evidence of enhanced VWF-mediated platelet adhesion in atherosclerosis-prone areas was demonstrated using contrast ultrasound molecular imaging.22 In collaborative studies, we have also shown that the amount of VWF and platelets detected on the vessel wall correlated with the atherosclerotic plaque burden.22,39 This study provides a new mechanism that links VWF with atherosclerosis: high levels of LDL, by promoting VWF fiber formation on the vessel wall and incorporating itself into VWF fibers, not only enhances the attachment of platelets and delivery of platelet-derived proatherogenic molecules but also enhances recruitment and delivery of the atherogenic LDL to the vessel wall.
There is evidence that elevated levels of VWF increase the risk of venous thromboembolism,40 although no correlation of lipids with venous thrombosis was found by Mendelian randomization.41 This may be caused by a mechanism independent of VWF self-association because VWF self-association requires shear stress, whereas venous clots usually form in areas of low shear stress or stasis, where self-association is expected to be minimal.
The balance between the 2 lipoproteins is also likely to influence the course of acute and chronic diseases affecting the microcirculation. High plasma levels of LDL greatly enhanced the formation of large, loose platelet aggregates in the microcirculation, slowing the blood flow and frequently embolizing downstream, potentially occluding smaller vessels (supplemental Video 6). This phenomenon may shed light on several published observations. Previously, deficiency of a prostacyclin carrier was proposed as a potential cause of TTP.42 This carrier was later identified as ApoA-I.43 Although deficient prostacyclin stabilization was subsequently shown not to be the cause of TTP,44 the association of low HDL with TTP has not been adequately explained.5 Similarly, risk factors for severe COVID-19, which is often accompanied by TMA,45,46 include those that are generally associated with dyslipidemias, such as obesity, diabetes, and hypertension, among others.47 However, in severe COVID-19 the lipoprotein profiles become further deranged48 and may not reflect their roles as a priori predictors of disease severity.
Lipoprotein regulation of VWF may also influence the course of other acute and chronic disorders without an obvious link to the vasculature, including multiple sclerosis (MS). Swank et al noted that MS was most prevalent in geographical regions with the highest animal fat consumption.49 He postulated that fat consumption triggered and accelerated MS. His group monitored microvascular blood flow in the cheek pouch of hamsters put on a high-fat diet for several days and heavy cream (35% fat) on the day of the experiment.25,50 They found that blood flow in the arterioles, capillaries, and venules slowed considerably, even stopping in some vessels at the peak of lipemia, an effect that persisted until the lipemia resolved. They observed erythrocyte rouleau formation and erythrocyte-platelet aggregation and noted that “on a few occasions, thin viscous strands could be seen attaching them together.” They observed a concomitant decrease in the platelet count, which was recovered when the lipemia subsided. Subsequently, they used oxygen probes to demonstrate that brain oxygenation dropped during the peak of the lipemia,51 implicating an impairment in the cerebral circulation. These observations led to the development of the Swank diet (low saturated fat) as an adjunct therapy for MS, a diet being prescribed today.52,53
Another phenomenon that may involve imbalanced lipoprotein regulation of VWF function was highlighted in a report by Wu et al,54 who studied microvascular perfusion in 8 patients with severe hypercholesterolemia before and after apheresis to remove LDL. Apheresis resulted in a great decrease in the plasma LDL concentration and an acute increase in myocardial perfusion and improved left ventricular function. A similar mechanism may account for the myocardial no-reflow phenomenon, in which reestablishing flow in a blocked coronary artery fails to reestablish downstream microvascular blood flow. Golino et al26 showed that rabbits with hypercholesterolemia subjected to transient coronary ligation had myocardial infarcts that were twice the size of those of control rabbits with no hypercholesterolemia,55 accompanied by a 4.5-fold increase in the nonreperfused zone, implicating microvascular obstruction associated with acute hypercholesterolemia. This group later showed that platelets were involved in the no-reflow phenomenon in hypercholesterolemic rabbits, because platelet depletion before coronary ligation reduced infarct size by >50% and reduced the nonreperfused zone to the size seen in control rabbits. Thus, platelets are involved in the effect of elevated LDL levels on the microcirculation, further implicating the LDL-VWF interaction.
Our recent study also demonstrated that hypercholesterolemia in the setting of ADAMTS13 deficiency greatly accelerated stenotic valve narrowing in a mouse model of aortic stenosis.56
The interaction of VWF with LDL was investigated by Cao et al who concluded that LDL reduced platelet adhesion to activated endothelial cells and that LDL increased the cleavage of VWF by ADAMTS13 and/or reduced VWF-VWF lateral aggregation.57 Our results are in disagreement with those conclusions. Using a similar tube-vortex approach, these investigators analyzed the VWF remaining in solution but did not assess the extent of VWF deposition on the tube surface, thus overestimating the extent of ADAMTS13-mediated cleavage. We recognize that shear-induced VWF cleavage occurs but find no evidence that it is enhanced by LDL. Furthermore, if LDL enhanced VWF cleavage, we should have observed a reduced large multimer content and increased peptides derived from cleaved VWF in the plasma of patients with hypercholesterolemia. Both analyses showed insignificant differences between normal and plasma of patients with hypercholesterolemia, providing no evidence of LDL-enhanced cleavage of VWF in vivo. In addition, imaging studies of mice with hypercholesterolemia also showed increased VWF-dependent platelet adhesion to the endothelium,22 indicating that endogenous ADAMTS13 could not overcome the prothrombotic effect of elevated LDL levels on platelet-endothelium interactions in vivo.
Our studies suggest that HDL and LDL bind directly to VWF, although we have not identified the binding sites. We hypothesize that the binding of the lipoproteins to VWF may not be competitive and favor a scenario in which binding of LDL to VWF lowers the shear threshold for exposing a self-association site that HDL can block. If true, it should be possible to reach an HDL concentration in which self-association would be inhibited even at the highest LDL concentrations. It is unlikely, however, that the high concentrations of HDL needed could be reached physiologically. It is also possible that HDL interacts directly with LDL under shear,58 reducing the effectiveness of LDL in promoting VWF self-association.
The self-association site within VWF has not been identified, but evidence suggests it is within the VWF A2 domain,59 which is most sensitive to unfolding by tensile force, such as shear stress. Unfolding is not only required for self-association but also for ADAMTS13-mediated cleavage. Structurally, the A2 domain belongs to the VWF A domain family, which is homologous to the I domains of several integrin α chains.60 The canonical structure of these domains encompasses a Rossmann fold, which contains a central hydrophobic core of β sheets surrounded by amphipathic α helices.61 Unlike the A1 and A3 domains of VWF, A2 lacks the disulfide bond that links the N- and C-termini of the domain, enabling it to be unfolded by lower tensile force to expose the hydrophobic core to the aqueous environment. We postulate that these hydrophobic regions either find similar regions on another VWF multimer to self-associate or are bound by HDL to block self-association. We also posit that LDL lowers the thermodynamic threshold for A2 unfolding. There is also evidence that the A2 domain can interact with the A1 domain under static conditions,62 and sites other than A2 may also contribute to VWF self-association.
In summary, our study demonstrates that LDL enhances the prothrombotic potential of VWF, predisposing to both macrovascular and microvascular dysfunction and suggests that new strategies to prevent microvascular thrombosis or tissue hypoperfusion might benefit from aggressive lowering of LDL or otherwise decreasing the LDL-to-HDL ratio.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute R01HL137991 (D.W.C.), R56HL131946 (D.W.C.), R35HL145262 (J.A.L.), R01HL117639 (J.A.L.), R01HL132985 (N.P., S.F.), R01HL136373 (N.P.), R01HL078610 (J.R.L.), R01HL130046 (J.R.L.); NIH Office of the Director grant P51OD011092 (J.R.L.); grant 18-18HCFBP_2-0009 (J.R.L.) from NASA; grant JP201860005 (K.O.) from Japan Society for the Promotion of Science (Overseas Research Fellowships); Murdock Charitable Trust Grant SR-201812420 (X.F.); and institutional funds from Bloodworks Northwest.
Authorship
Contribution: D.W.C., J.A.L., and J.R.L. conceived the study, designed experiments, analyzed data, and wrote the manuscript; J.C. designed and performed experiments, analyzed data, and aided in writing the manuscript; K.P., K.O., R.A., F.N., A.S.J., M.L., J.L., J.H., N.R., Y.W., and X.F. performed experiments and analyzed data; and N.P. and S.F. provided advice and reagents and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for S.F. is Regeneron Pharmaceuticals, Tarrytown, NY.
Correspondence: Dominic W. Chung, Bloodworks Research Institute, 1551 Eastlake Ave E, St 100, Seattle, WA 98102; e-mail: chung@bloodworksnw.org; and José A. López, Bloodworks Research Institute, 1551 Eastlake Ave E, St 100, Seattle, WA 98102; e-mail: josel@bloodworksnw.org.
References
Author notes
Original data and protocols related to this publication are available on request from the corresponding author, Dominic W. Chung (chung@bloodworksnw.org).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.


![VWF self-association at various HDL and LDL levels. Bio-VWF (5 μg/mL) in 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.4 was vortexed for 90 minutes at room temperature in the presence of increasing concentrations of HDL (A) or in the presence of either 1.2 or 2.4 mg/mL of HDL and increasing concentrations of LDL (B). The Bio-VWF remaining in solution was measured using ELISA. Bio-VWF in tubes not vortexed served as control (100%) (n = 3 in [A], n = 4 in [B]). (C) Citrated pooled human plasma (50%) in 10 mM EDTA was sheared by vortexing for 3 hours. VWF remaining in solution was measured using ELISA and expressed as a percentage of the VWF in parallel samples not exposed to shear stress (n = 3). The ratios of LDL to HDL are shown in both cholesterol ratios (LDL-C to HDL-C) and total particle weight ratios (LDL to HDL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/13/10.1182_blood.2023019749/2/m_blood_bld-2023-019749-gr1.jpeg?Expires=1770229865&Signature=sCmpO3mx3NSN~X1XiiQj2n2QVt9p1PrLFQ4B2ETCiHSN-0Vn2f0lRFgpfl0bYpuA5-tCKQbbpSAaIuQvbVwT6yvmPhoD3wHBPToiLRpEjkrzuD27U-ZIGd-LNGEABPIjrnQFDIxa5sA4mfHm~XAfyR5Lf6H-akYUo59S84Np~n2SMrrWjo5iNzdkdyluW1zoPpJk7Tw7tHBOIDFZILlAnBR5oLFC5WXyJm4ZvrDzYBLO1eHHgn0StsYogEbtlt-7ltQmGNz6Y3e3aEzcea1Q43C7tCO1uBC6HafdIDxJF~L0FoAkYEy3ZjTFO635qGpgb-Yu7FgT8PzB6JCpHPk9TQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)