Introduction
Smoldering multiple myeloma (SMM) is a heterogeneous condition which includes patients with varying risk of progression to active multiple myeloma (MM). The current risk stratification model is based on biomarkers reflecting disease burden at the time of diagnosis, without regard to evolution of biomarkers over time. In this study, we evaluated the dynamic changes in the monoclonal protein (MP) concentration and the free light chain ratio (FLCr), and their relation to risk of progression from SMM to MM.
Methods
We included all SMM patients managed at Memorial Sloan Kettering Cancer Center between 2002 and 2019, with follow up until end of 2022. Date of diagnosis and progression, as well as baseline laboratory, pathology and imaging data were manually reviewed. Serial FLC and MP were obtained through computerized data extraction. Diagnoses of SMM and MM were defined according to IMWG 2014 criteria and/or starting MM directed therapy. Time to progression (TTP) was assessed using the Kaplan-Meier method, with log-rank tests for comparison between groups. We used multivariate Cox regression to estimate the risk of progression with hazard ratios (HRs) and 95% confidence intervals (CIs).
Results
A total of 398 patients were included in the study; the median age was 64 years and 55% were men. At baseline, the median M-protein was 1.3 g/dL, and the median FLCr was 9.8. The overall median TTP for the cohort was 94 months during a median follow up time of 65 months.
First, we stratified patients into quintiles (Q1-Q5) based on baseline M-protein and FLCr, with the highest risk of progression seen in Q5 for M-protein (defined as MP ≥2.2 g/dL, median TTP 29 months [95% CI: 24-64]) and Q5 FLCr (defined as FLCr ≥26, median TTP 36 months [29-62]), respectively (p<0.001).
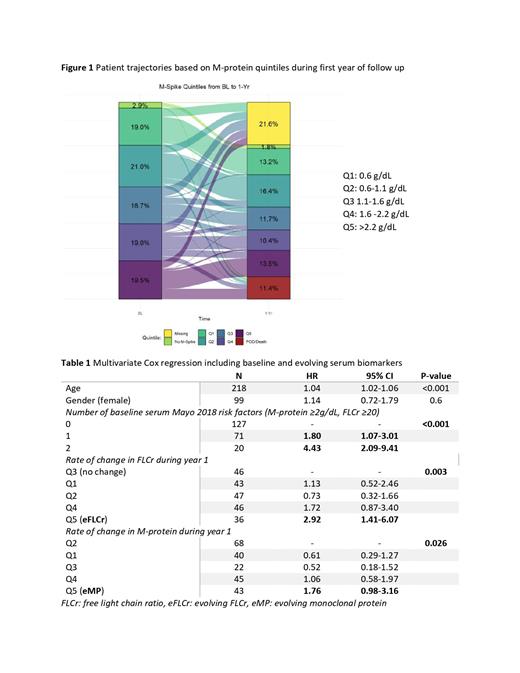
We compared patient trajectories during the first year of follow up based on the baseline quintiles. Interestingly, among patients who progressed from SMM to MM during the first year (11%, n=42/369), only 43% had a baseline M-protein ≥2.2 g/dL (Q5) and 43% had a FLCr ≥26 (Q5). In fact, among those who progressed, 29% had a baseline M-protein <1.6 g/dL (Q1-Q3), and 26% had baseline FLCr <11.3 (Q1-Q3). The patient trajectories during the first year of follow up based on the M-protein level are characterized in Figure 1.
We then analyzed changes in M-protein and FLCr during the first year, and stratified patients into quintiles based on the rate of change. We defined evolving M-protein (eMP) and evolving FLCr (eFLCr) based on the levels of the top quintile for each group, which was an increase in ≥0.3 g/dL for eMP and ≥50% increase for eFCLr. Patients with eMP had an increased risk of progression with a median TTP of 22 months (17-not reached [NR]) after the first year (p<0.001). Patients with eFLCr had a median TTP of 48 months (21-116) after the first year (p<0.001).
Recognizing that patients with high baseline M-protein level and FLCr also may have a greater increase, we developed a multivariate model adjusting for baseline levels of M-protein and FLCr. In the multivariate model, we included age, gender, and baseline M-protein ≥2g/dL and FLCr ≥20 from the Mayo 2018 risk score, as well as eMP and eFLCr. Adjusting for baseline risk, patients with eMP (Q5) had a HR of 1.8 (0.98-3.16) of progression and patients with eFLCr (Q5) had a HR of 2.9 (1.4-6) of progression using patients with little to no change in M-protein or FLCr as reference (Table 1).
Finally, we did a subgroup analysis evaluating eMP and eFLCr in each baseline risk group separately. Patients with no serum risk markers per Mayo-2018 at baseline (i.e. MP <2g/dL, FLCr <20, n=127) who had both eMP (≥0.3 g/dL increase) and eFLCr (≥50% increase), had a median TTP of 25 months (0.42-NR), patients who had either eMP or eFLCr had a median TTP of 100 months (42-NR), whilst patients with neither eMP nor eFLCr had a median TTP of 201 months (130-NR), (p<0.001).
Conclusion
In summary, we found that when adjusting for baseline M-protein level and FLCr, evolving changes in M-protein and FLCr were associated with a higher risk of progression from SMM to MM. Interestingly, for patients with low risk by baseline stratification (M-protein <2g/dL and FLCr <20), the presence of eMP (≥0.3 g/dL increase) and eFLCr (≥50% increase) was associated with a median TTP of 25 months, similar to those with a baseline high risk. Thus, in addition to baseline disease burden, dynamic disease evolution should be considered in the risk stratification for patients with SMM.
Disclosures
Korde:Amgen, Janssen, Epizyme, AbbVie: Research Funding; Janssen: Other: Advisory Board; CCO, OncLive, Intellisphere, Remedy Health: Consultancy. Mailankody:Optum Oncology: Consultancy; MJH Life Sciences: Honoraria; Bristol Myers Squibb: Research Funding; Fate Therapeutics: Research Funding; Janssen Oncology: Research Funding; Takeda Oncology: Research Funding; Physician Education Resource: Honoraria; Allogene Therapeutics: Research Funding; OncLive: Honoraria; Janssen Oncology: Consultancy; Legend Biotech: Consultancy; Caribou Therapeutics: Research Funding. Lesokhin:ArcellX: Consultancy; Janssen: Honoraria, Research Funding; Bristol Myers Squibb: Research Funding; Pfizer: Honoraria, Research Funding. Hassoun:Celgene, Takeda, and Janssen Pharmaceuticals: Research Funding. Shah:M and M Labs: Research Funding; Sanofi: Other: Advisory Board; Sabinsa: Research Funding; Plantable: Research Funding; Janssen: Consultancy, Other: Advisory Board, Research Funding; Bristol Myers Squibb: Consultancy, Other: Advisory Board, Research Funding; C4 Therapeutics: Research Funding. Tan:Takeda: Research Funding; Janssen: Current Employment, Honoraria, Research Funding; Sanofi: Honoraria. Lahoud:MorphoSys Inc, Kite: Consultancy. Landau:Karyopharm, Pfizer, Juno, Prothena, Caelum Biosiences, Legend Biotech, Takeda, Janssen, Nexcella: Honoraria; Alexion Pharmaceuticals, Takeda, Janssen, Prothena, Protego: Research Funding. Shah:ArcellX: Other: DSMB; Amgen: Research Funding; Beyond Spring: Research Funding; BMS: Research Funding; Janssen: Research Funding. Scordo:CancertNetwork (Intellisphere LLC): Honoraria; Angiocrine Bioscience, Inc.: Research Funding; Medscape, LLC: Honoraria; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding. Landgren:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Adaptive: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Theradex: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Membership on independent data monitoring committees. Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Usmani:GSK: Membership on an entity's Board of Directors or advisory committees, Research Funding; K36 Therapeutics: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Moderna: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; EdoPharma: Membership on an entity's Board of Directors or advisory committees; SkylineDX: Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Array Biopharma: Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; TeneoBio: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; SecuraBio: Membership on an entity's Board of Directors or advisory committees; Bristol Meyer Squibb: Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hultcrantz:Curio Science LLC, Intellisphere, Bristol Myer Squibb, GlaxoSmithKline: Honoraria; Amgen, Daiichi Sankyo, GlaxoSmithKline: Research Funding.