Abstract
No randomized study comparing the effect of combined ticlopidine and aspirin therapy versus each drug alone in reducing poststenting thrombotic complications has been performed. To compare these three antiplatelet regimens versus placebo, we conducted a double-blind randomized study using an ex vivo model of thrombosis. Sixteen healthy male volunteers were assigned to receive for 8 days the following four regimens separated by a 1-month period: aspirin 325 mg/d, ticlopidine 500 mg/d, aspirin 325 mg/d + ticlopidine 500 mg/d, and placebo. At the end of each treatment period, native nonanticoagulated blood was drawn directly from an antecubital vein over collagen- or tissue factor (TF)-coated coverslips positioned in a parallel-plate perfusion chamber at an arterial wall shear rate (2,600 s−1 ) for 3 minutes. Thrombus, which formed on collagen in volunteers treated by placebo, were rich in platelets and poor in fibrin. As compared with placebo, aspirin and ticlopidine alone reduced platelet thrombus formation by only 29% and 15%, respectively (P > .2). In contrast, platelet thrombus formation was blocked by more than 90% in volunteers treated by aspirin + ticlopidine (P < .01v placebo or each treatment alone). Furthermore, the effect of the drug combination therapy was significantly larger than the sum of the two active treatments (P < .05). Thrombus, which formed on TF-coated coverslips in volunteers treated by placebo, were rich in fibrin and platelets. Neither of the three antiplatelet treatments significantly inhibited fibrin deposition and platelet thrombus formation on this surface (P > .2). Thus, the present study shows that combined aspirin and ticlopidine therapy dramatically potentiates the antithrombotic effect of each drug alone, but that the antithrombotic effect of the combined treatment depends on the nature of the thrombogenic surface.
© 1998 by The American Society of Hematology.
THE ROLE OF PLATELETS in arterial thrombosis has been well-established during the last decades. As a result, platelet inhibitor therapy has become of considerable interest for prevention and treatment of acute and chronic arterial diseases. Among currently available antiplatelet drugs, aspirin blocks platelet production of thromboxane A2 and ticlopidine inhibits adenosine diphosphate (ADP)-induced platelet aggregation. When used as single antithrombotic agents, these drugs have a moderate clinical efficacy.1 This may be due to the fact that aspirin and ticlopidine interfere with only one of the various pathways of platelet activation. Therefore, to improve the antithrombotic effect, it has been suggested to combine these two drugs. Thus, ticlopidine potentiated the antithrombotic effect of aspirin in a recent experimental study performed in rats.2 Furthermore, this combined antiplatelet therapy was very potent in reducing cardiac events and vascular complications after coronary artery stent placement as compared with conventional anticoagulant therapy.3However, no clinical or experimental study has randomly compared in humans the antithrombotic effect of the combination of aspirin and ticlopidine to each drug alone.
The antithrombotic effect of drugs can be experimentally investigated in humans using an ex vivo model of thrombogenesis, which closely mimics relevant clinical situations.4-8 In this model, native blood is drawn from healthy volunteers through a parallel-plate chamber device where it interacts, in well-established flow conditions, with a thrombogenic surface. Blood flow conditions mimic wall shear rates encountered in moderately stenosed arteries (2,600 s−1). Two relevant thrombogenic molecules, which are present in atherosclerotic plaques and primarily responsible for thrombus formation,9,10 are exposed to blood, ie, collagen and tissue factor (TF). The efficacy of antithrombotic drugs is determined by quantifying the respective thrombus content in platelets and fibrin by immunoenzymatic methods. This model has been used to investigate the antithrombotic effect of aspirin, linotroban, a thromboxane A2 receptor inhibitor, and clopidogrel, respectively.11-14 The latter two drugs moderately reduced thrombus formation, but no significant effect was found with aspirin. Using this ex vivo model of acute initial thrombus formation, we designed a double-blind, randomized study with blood from healthy volunteers to compare the antithrombotic effect of three different antiplatelet regimens versus placebo: either combined aspirin + ticlopidine therapy, or aspirin or ticlopidine alone.
MATERIALS AND METHODS
Subjects
The study population consisted of healthy white male volunteers aged 20 to 30 years. They had no history or clinical sign of any disease and did not take any medication known to affect blood coagulation or platelets during the study period. The volunteers smoked less than 10 cigarettes per day, and they did not smoke the day of the perfusion experiments. Clinical chemistry, hematologic, and hemostatic laboratory values were within the normal ranges. All subjects gave written, informed consent to the protocol, which was approved by the local Human Subjects Committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale, Toulouse). All eligible volunteers were randomly assigned to one of the four sequences of treatment, as indicated below.
Study Design
This monocentric, randomized, controlled, double-blind study was performed in the Center for Clinical Investigation at Hôpital Purpan, Toulouse, France. After selection for the trial, each volunteer satisfying the inclusion and exclusion criteria received one of the four tested regimens (Table 1): either (I) placebo for 8 days, or (II) placebo during 5 days associated with aspirin 325 mg once daily the last 3 days, or (III) ticlopidine 250 mg twice daily plus placebo for 8 days, or (IV) ticlopidine 250 mg twice daily for 8 days associated with aspirin 325 mg once daily during the last 3 days. These periods of administration were chosen to obtain a full pharmacologic effect. Aspirin was given for a shorter period because its pharmacologic effect is more quickly reached than that of ticlopidine and because, for safety reasons, the healthy volunteers enrolled in this study had to receive the combined treatment for the shortest possible period. The four regimens were given separately with a wash-out period of 3 to 6 weeks between each of them. All drugs and placebo were supplied by Sanofi Recherche (Toulouse, France) under blister indicating the day and time of intake. On the eighth day of treatment, the subjects were requested to come to the study center to be examined and rest 1 or 2 hours before blood donation. All adverse effects were recorded and appropriate follow-ups were monitored. Premature withdrawal from the study, for any reason (adverse effect, wish of the subject, lack of compliance), led to replacement of the subject by another volunteer who followed the same sequence so that 16 complete case reports could be available for analysis at the end of the study.
Preparation of thrombogenic surfaces.
The thrombogenic molecules were coated on Thermanox plastic coverslips (Miles Laboratories, Naperville, IL) previously washed in 70% ethanol and rinsed five times in sterile water. Equine collagen (Collagenreagent Horm; Nycomed, Munchen, Germany) was spray-coated onto plastic coverslips to a final density of 0.5 μg/cm2. The collagen-coated coverslips were stored at room temperature for 15 to 20 hours before use as described previously.4,5,8 TF, purified from human placenta (Thromborel; Behring, Rueil-Malmaison, France), was used as described previously.6 8 Dilution of 1:50 was prepared in coating buffer (0.1 mol/L sodium carbonate, pH 9.5). Thermanox plastic coverslips were incubated in 2 mL of the Thromborel dilution for 17 hours at 4°C. The TF-coated coverslips were rinsed five times with phosphate-buffered saline (PBS; Seromed, BiochromKG, Berlin, Germany) and stored for less than 7 hours at 4°C before use.
Perfusion experiments.
Two perfusion experiments were performed after each period of treatment, with collagen and TF as thrombogenic surface, respectively. Perfusion experiments were performed with a parallel-plate perfusion chamber device at 37°C.5,7,8 After blood sample collections (see below), native blood was drawn directly from an antecubital vein of the volunteers through a 19G infusion set (Ohmeda, Helsingburg, Sweden) over a collagen-coated coverslip positioned in the parallel-plate perfusion chamber. The blood flow rate was maintained at 10 mL/min by a peristaltic roller pump (Multiperpex LKB, Pharmacia, St-Quentin-en-Yvelines, France) placed distal to the chamber. Given the cross-sectional dimension of the blood flow channel of the perfusion chamber, the wall shear rate was 2,600 s−1, which corresponds to that encountered in moderately stenosed arteries. The blood perfusion experiment lasted for 3 minutes and was followed by a 30-second perfusion of PBS at the same flow rate to wash out blood from the flow channel. The coverslip covered by thrombotic deposits was removed from the chamber and divided into two equal parts parallel to the direction of the blood flow, as described previously.8One half of the thrombotic deposit was placed in a plasmin solution and processed as described below. The other half was immersed into freshly prepared fixation solution (2.5% glutaraldehyde in 0.1 mol/L cacodylate, pH 7.4) at 4°C for 90 minutes, and stored in 0.1 mol/L cacodylate 7% sucrose at 4°C until embedded in Epon. A second perfusion experiment was subsequently performed with blood directly drawn from a contralateral cubital vein over a TF-coated coverslip.
Evaluation of the Efficacy of the Different Drug Regimens
The efficacy of each drug treatment was evaluated by morphometry, immunology, and by the determination of platelet activation and thrombin formation downstream to the site of thrombus formation. As previously described, three methods of quantification were used because they give complementary information about thrombus size, thrombus composition, and mechanisms of thrombus formation.8
Morphometrical determination of thrombotic deposits.
Microscopic evaluation of thrombotic deposits was performed on epoxy embedded semithin sections (1 μm thick) stained with toluidine blue and basic fuchsin, as previously described.8,15 The sections were prepared at an axial position of 2 mm downstream from the upstream edge of the coverslip and perpendicular to the direction of the blood flow. Standard morphometry,15 performed by light microscopy at 1,000 × magnification, was used to quantify the percent coverage with platelets adherent to collagen or fibrin (% platelets). These morphometric evaluations were performed at 10-μm intervals along the surface by moving the section along an eye-piece micrometer in the microscope ocular.
Immunological determination of fibrin and platelet deposition.
Fibrin deposition was quantified by immunologic determination of fibrin degradation products of plasmin-digested thrombi, as described previously.16 17 After perfusions with blood and PBS, the thrombus was immediately incubated in 2 mL of a plasmin solution (Chromogenix, Mölndal, Sweden, 0.7 IU/mL, in Tris-buffered saline, pH 7.4) for 30 minutes under gentle shaking and at 37°C. Plasmin digestion was stopped by aprotinin (2,000 KIU/mL, Sanofi, Gentilly, France). The solution was centrifuged (4°C, 4,300g, 15 minutes) and the supernatant frozen at −80°C for measurement of fibrin degradation products and P-selectin levels (see below). Fibrin degradation products were measured using an immunoenzymatic assay (Asserachrom D-Di; Stago, Asnières, France). The amount of deposited fibrin is directly determined from the levels of fibrin degradation products expressed in fibrin equivalent units as indicated by the manufacturer: this unit corresponds to the quantity of clotted fibrinogen that leads to the observed fibrin degradation products level.
Platelet deposition was quantified by measurement of a specific platelet α granule membrane protein, P-selectin.8 After centrifugation of the plasmin-digested thrombus, the pellet was dissolved in 400 μL of a lytic buffer, three times frozen and thawed, and then sonicated (4°C, 20 kHz) for 270 seconds. The lytic buffer is made of PBS containing 1% Triton X-100 (Merck, Chelles, France), 16 mmol/L octyl-β-D glucopyranoside (Boehringer Mannheim, Meylan, France), 1 mmol/L EDTA (Merck), 20% sodium azide (Merck), 10 μmol/L pepstatin A (Sigma, Saint-Quentin-Fallavier, France), 10 μmol/L leupeptine (Sigma), 100 KIU/mL aprotinin, 0.1 mmol/L phenylmethyl sulfonyl fluoride (PMSF) (Sigma). All samples of dissolved pellets were stored at −80°C until assayed for P-selectin measurement by immunoenzymoassay (Bender MedSystems, Vienna, Austria). The level of P-selectin was measured both in the dissolved pellet and in the supernatant of the plasmin-digested thrombus. Total number of platelets deposited was calculated from the amount of P-selectin present in the thrombus and that present in corresponding nonactivated platelets of each blood donor determined before the ex vivo perfusion experiment. Results were expressed as number of platelets deposited/cm2. The area of coverslip surface exposed to the blood flow is 0.9 cm2.
Determination of platelet activation and thrombin formation.
Platelet activation, thrombin generation, and fibrin formation were determined by measuring plasma levels of β thromboglobulin (βTG), thrombin-antithrombin complexes (T-AT), and fibrinopeptide A (FPA), respectively. βTG, T-AT, and FPA were measured in blood (3.2 mL) collected in 0°C precooled tubes or syringes containing a mixture (0.8 mL) of platelet inhibitors and anticoagulants (sodium citrate, citric acid, theophylline, adenosine, dipyridamole, heparin, and aprotinin), as described previously.7 Blood samples were collected first at the flow inlet of the chambers at the beginning of the perfusion experiment to determine whether the different drug regimens influenced the basal plasma levels of markers of platelet activation and thrombin formation. Then, to measure the platelet activation and thrombin formation secondary to blood interaction with the thrombogenic surfaces, blood samples were collected at the flow outlet of the chambers between 2.5- and 3-minute perfusions, by a syringe pump (Harvard Apparatus, South Natick, MA), as previously described.7 Blood samples were immediately centrifugated (4,300g, 4°C, 30 minutes) and aliquots of plasma were stored at −80°C until assayed. The plasma concentrations of βTG, FPA, and T-AT were measured by immunoenzymoassays (Assera-βTG and Assera-FPA, Stago; Enzygnost-T-AT, Behring, respectively).
Other Laboratory Procedures
Red blood cell, leukocyte and platelet count, hemoglobin, and hematocrit were measured by an electronic counting device (Model S plus; Coulter Electronics, Hialeah, FL) during and after each period of treatment. Subjects’ compliance was checked by performing platelet aggregation tests: blood was collected into a citrated vacutainer (Becton Dickinson, ref 367704, Meylan, France) containing 0.5 mL of 0.129 mol/L trisodium citrate for 4.5 mL of blood. Platelet-rich plasma was obtained after a centrifugation at 150g, 15 minutes, at room temperature and platelet-poor plasma was obtained after a second centrifugation at 1,500g, 15 minutes. Platelet count was adjusted to 250 × 109/L by appropriate dilution of the platelet rich-plasma with autologous platelet-poor plasma. Platelet aggregation was performed with a platelet aggregometer (Coulter Electronics) at 37°C and 1,000 rpm following calibration with platelet-rich plasma (10% optical transmission) and platelet-poor plasma (90% optical transmission). The aggregating agents were ADP (2.5 and 5 μmol/L final concentration; Stago), equine collagen (3.3 and 10 μg/mL final concentration; Nycomed) and arachidonic acid (1 mmol/L final concentration, BioData Corp, Horsham, PA). The maximum amplitude of platelet aggregation was measured and expressed as a percentage of the difference between platelet-rich plasma and platelet-poor plasma.
Statistical Analysis
Results were expressed as mean ± 1 standard error of mean (SEM). A four-treatment, four-period, cross-over design, called William’s design and an analysis of variance (ANOVA) mixed model (unstructured matrix when compound symetry not appropriate) with terms for subject, period, first order carry-over, and treatment were used for statistical analysis. In either situation, the 95% confidence interval for each treatment difference has been calculated. In all statistical hypothesis tests, the level of significance P was < .05.
RESULTS
Study Population
Twenty-one male, white subjects, ages 20 to 30 years (mean age, 24 years) were enrolled in the study and randomly assigned to the different treatments from February 1996 through July 1996. Five stopped the trial prematurely. In 2 cases, the trial was stopped because of an error in the treatment allocation and a technical problem in the perfusion experiment, respectively. In 3 other cases, the trial was stopped for adverse events: 2 subjects had low neutrophil count (1.78 × 109/L and 1.75 × 109/L under ticlopidine and aspirin treatment, respectively; normal values: 2.0 to 7.0 × 109/L) and 1 had pruritus with ticlopidine. Other minor adverse events, notably bleeding and gastrointestinal disorders, were rare, very moderate, and they did not lead to the interruption of treatment. In particular, the bleeding disorders were 1 purpura, 5 epistaxis, 2 rectorragia, and they were evenly distributed within the three groups with active treatment (3 bleeding disorders with aspirin, 3 with ticlopidine, and 2 with aspirin + ticlopidine). Sixteen volunteers completed the study according to the protocol.
Effect of Treatment on Platelet Aggregation
Platelet aggregation was evaluated on blood samples drawn from volunteers before each perfusion experiment. Results are shown in Table 2. Ticlopidine inhibited ADP-induced (P < .01), but not collagen-induced platelet aggregation. In contrast, aspirin inhibited collagen-induced (P < .01), but not ADP-induced platelet aggregation. Collagen-induced platelet aggregation was significantly more inhibited by the combination aspirin + ticlopidine than by aspirin alone (72% and 43% reductionv 48% and 16% reduction as compared with placebo at 3.3 and 10 μg/mL, respectively, P < .01). In contrast, the inhibitory effect of ticlopidine on ADP-induced platelet aggregation was not enhanced by aspirin. Finally, aspirin, alone or associated with ticlopidine, fully inhibited arachidonic acid-induced platelet aggregation in all 16 volunteers.
Effect of Treatment on Baseline Level of Hemostatic System Activation
Baseline values of plasma markers of platelet (βTG) and coagulation activation (T-AT, FPA) were within normal ranges in the volunteers treated by placebo (24.5 ± 2.9, 2.8 ± 0.2, and 2.0 ± 0.4 μg/mL, respectively). These values remained unchanged when the volunteers were treated by aspirin, ticlopidine, or aspirin + ticlopidine (P > .2, data not shown).
Effect of Treatment on Thrombus Formation on Collagen
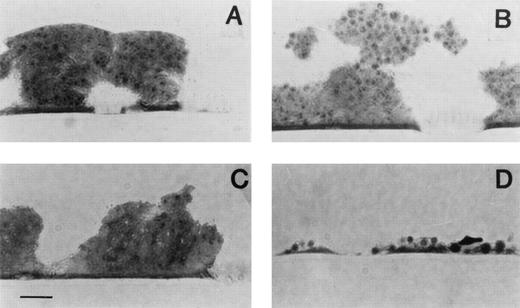
The respective effects of the three antithrombotic treatments on collagen-induced thrombus formation are shown in Fig 1. Representative light micrographs are shown in Fig 2. In volunteers treated with placebo, and as previously found,8 thrombi formed on collagen were rich in platelets and poor in fibrin. Compared with placebo, aspirin and ticlopidine alone had a modest antithrombotic effect. Aspirin and ticlopidine decreased platelet deposition by 29% and 15%, respectively, (P > .2) and fibrin deposition by 42% (P = .01) and 37% (P = .04), respectively. In contrast, platelet thrombus formation and fibrin deposition were almost totally blocked in all 16 volunteers treated by aspirin + ticlopidine (P < .01 v placebo or each treatment alone). Furthermore, the combination of aspirin + ticlopidine was significantly more efficient in reducing thrombus formation than the sum of the two active treatments (P < .05). Interestingly, whereas platelet adhesion, which represents the percent of surface covered with platelets, was enhanced by aspirin (24% enhancement as compared with placebo, P < .04, Table 3), there was a slight, but nonsignificant, decrease of platelet adhesion in volunteers treated by aspirin + ticlopidine (17% reduction, P = .13).
Effect of antiplatelet treatment on deposition of platelets (□) and fibrin (▪) on collagen-coated coverslips evaluated by immunoenzymology. The surface was exposed for 3 minutes to nonanticoagulated blood at a shear rate of 2,600 s−1 from volunteers given either aspirin (ASA), ticlopidine (T), or the combination of both (ASA + T) or placebo (P). Values are means ± SEM (n = 16). *P < .05, **P < .01 versus placebo.
Effect of antiplatelet treatment on deposition of platelets (□) and fibrin (▪) on collagen-coated coverslips evaluated by immunoenzymology. The surface was exposed for 3 minutes to nonanticoagulated blood at a shear rate of 2,600 s−1 from volunteers given either aspirin (ASA), ticlopidine (T), or the combination of both (ASA + T) or placebo (P). Values are means ± SEM (n = 16). *P < .05, **P < .01 versus placebo.
Representative light micrographs of blood-collagen interactions. The surface was exposed at a shear rate of 2,600 s−1 during 3 minutes to nonanticoagulated blood drawn from volunteers given either placebo (A), aspirin (B), ticlopidine (C), or the combination of both (D). The sections were prepared perpendicular to the direction of the blood flow. Note that the collagen coat is not visible because the staining procedure does not contrast the fibrils. Original magnification × 1,000. Bar represents 10 μm.
Representative light micrographs of blood-collagen interactions. The surface was exposed at a shear rate of 2,600 s−1 during 3 minutes to nonanticoagulated blood drawn from volunteers given either placebo (A), aspirin (B), ticlopidine (C), or the combination of both (D). The sections were prepared perpendicular to the direction of the blood flow. Note that the collagen coat is not visible because the staining procedure does not contrast the fibrils. Original magnification × 1,000. Bar represents 10 μm.
The plasma levels of markers of platelet activation (βTG), thrombin (T-AT), and fibrin (FPA) formation in volunteers treated by placebo were, respectively, 7, 1.5, and 3 times higher in effluent blood sampled distal to the site of thrombus formation than their respective baseline levels (Table 4). Aspirin, ticlopidine, and aspirin + ticlopidine prevented the release of β-TG in a comparable manner (P < .05). However, neither treatment significantly decreased distal levels of T-AT and FPA.
Effect of Treatment on Thrombus Formation on TF
The results of thrombi formed on TF after the three therapeutic regimens are shown in Fig 3. Thrombus, which formed on TF-coated coverslips in volunteers treated by placebo, were rich in fibrin and platelets, with platelets deposited almost exclusively on top of fibrin meshes (data not shown). Neither treatment significantly inhibited fibrin deposition or platelet thrombus formation on this surface (P > .2).
Effect of antiplatelet treatment on deposition of platelets (□) and fibrin (▪) on TF-coated coverslips evaluated by immunoenzymatic method. The surface was exposed at a shear rate of 2,600 s−1 during 3 minutes to nonanticoagulated blood drawn from volunteers given either aspirin (ASA), or ticlopidine (T), or the association of both (ASA + T) or placebo (P) according to the protocol. Values are means ± SEM (n = 16).
Effect of antiplatelet treatment on deposition of platelets (□) and fibrin (▪) on TF-coated coverslips evaluated by immunoenzymatic method. The surface was exposed at a shear rate of 2,600 s−1 during 3 minutes to nonanticoagulated blood drawn from volunteers given either aspirin (ASA), or ticlopidine (T), or the association of both (ASA + T) or placebo (P) according to the protocol. Values are means ± SEM (n = 16).
TF-dependent thrombus formation resulted in a much higher activation of coagulation, but also of platelets, than collagen-dependent thrombus formation: plasma levels of βTG, T-AT, and FPA were, respectively, 4, 42, and 55 times higher with TF than with collagen (P < .05) (Tables 4 and 5). Neither treatment prevented the formation of these markers of platelet and coagulation activation (P > .2).
DISCUSSION
Subacute and acute arterial thrombosis are main concerns, which have promoted a search for new antithrombotic regimens. The combination of aspirin and ticlopidine has been tested in several trials, notably after coronary artery stent placement.3,18-22 Most of the studies indicate a better efficacy3,20-22 of the antiplatelet drug combination on bleeding complications, hospital stay duration, and stent thrombotic closure rates. Nevertheless, the most recent study has suggested that stent thrombosis may be equally prevented by aspirin or aspirin + ticlopidine.19 Thus, the exact respective pharmacologic value of aspirin, ticlopidine, and the combined therapy remains unknown.
The primary goal of the present study was to quantify the antithrombotic effect of the combination of aspirin and ticlopidine on thrombus formation in an ex vivo model of human thrombogenesis and to compare it with the antithrombotic effect of aspirin and ticlopidine alone in a double-blinded and randomized manner. We demonstrate that the combination of ticlopidine and aspirin dramatically potentiated the antithrombotic effect of each drug alone: aspirin plus ticlopidine almost totally blocked thrombus formation on collagen substrate, whereas aspirin or ticlopidine alone were only modestly effective. However, the antithrombotic effect of the combination aspirin + ticlopidine depended on the nature of the thrombogenic surface, as it was totally ineffective in preventing arterial thrombus formation elicited by TF.
The ex vivo model of human thrombogenesis used in the present work allows the study of thrombosis directly in man under various and well-controlled blood flow conditions and on different types of relevant vascular thrombogenic surfaces.4-8 Both collagen and TF are present in human atherosclerotic plaques and both have been shown to be major determinants of thrombus formation at human atherosclerotic lesions after plaque disruption.9 10Furthermore, mechanisms involved in thrombus formation in relation to the antithrombotic efficacy of different antithrombotic regimens may also depend on these substrates. Therefore, we have chosen to study antithrombotic drugs by using these two major thrombogenic substrates because they give different and complementary information of antithrombotic efficacy and because they are relevant to various clinical situations, which are complicated by thrombosis. Shear conditions were controlled with wall shear rates of 2,600 s−1, mimicking those encountered in moderately stenosed coronary arteries.
Both morphometric and immunologic approaches were used to measure thrombus formation, because together they give optimal and complementary information about thrombus formation.8Morphometric analysis allows direct visualization of the thrombus (Fig2) and it allows the determination of platelet-surface interaction, ie, platelet adhesion (Table 3). Besides its simplicity, the immunologic methods allow quantitative measurements of both platelets and fibrin of the whole thrombi formed on the thrombogenic surface. The two methods were performed on the same specimen by dividing the thrombotic deposits into two equal large parts in parallel with the direction of the blood flow, as deposition of platelets and fibrin varies in an axial-dependent manner.23 24
Thrombi on the collagen substrate were rich in platelets, but poor in fibrin. This platelet thrombus formation was modestly prevented by aspirin, although not significantly different from the placebo group (P > .2). Comparable data from previous studies using the same thrombosis model are available, and these studies showed furthermore that the antithrombotic effect of aspirin increases with increasing shear rate.11,12 Ticlopidine shows a moderate antithrombotic effect, as well (P > .2). Comparable results were obtained with a newer and more potent thienopyridine derivative, clopidogrel.14 In this model, significant antithrombotic effect of clopidogrel on platelet thrombus formation at 2,600 s−1 appeared after a longer period of drug intake, ie, between 1 and 2 weeks of daily oral ingestion. The antithrombotic effect obtained in subjects treated by the combination aspirin + ticlopidine was quite remarkable, as platelet thrombus formation was almost totally blocked in all 16 volunteers treated by this therapeutic regimen. Statistical analysis indicated furthermore that there was a synergistic effect of the association because the effect was exceeding the sum of the effects of each treatment alone. Taken together, these results indicate that both ADP and thromboxane A2 play a major and complementary role in mediating collagen-induced platelet thrombus formation. However, to achieve a significant antithrombotic effect, one needs to block simultaneously both pathways, as either pathway is able to compensate the other one.
Aspirin + ticlopidine blocked thrombus formation (Fig 1), but had no effect on platelet adhesion (Table 3), indicating that different mechanisms are involved in these two subsequent processes of thrombus formation: ADP and thromboxane A2 are not involved in platelet adhesion, whereas they are important mediators of platelet aggregation. However, it is interesting to note that the total inhibition of platelet thrombus formation by aspirin + ticlopidine did not result in enhanced platelet adhesion. In the present study and in other works performed with comparable models, antithrombotic agents, which decreased platelet-platelet interactions, increased platelet adhesion.12-14,25 There is indeed a balance between the platelet supply to the reactive surface and the consumption of platelets by growing thrombi23,24: when the platelet consumption by the growing thrombi decreases, there is concomitantly an increase in the platelet concentration in the blood layers streaming adjacent to the collagen surface. This results in more platelets available for adhering to collagen. Two steps are necessary for an optimal platelet adhesion to collagen at this high shear condition: an initial reversible glycoprotein Ibα-mediated platelet adhesion subsequently followed by a glycoprotein IIb/IIIa activation, which allows to firmly tether platelets to the collagen surface through its interaction with adhesive proteins.26 Thus, it is possible that the simultaneous ingestion of aspirin and ticlopidine inhibited platelet activation, and consequently glycoprotein IIb/IIIa activation much more efficiently than aspirin alone, so that firm adhesion after the initial weak collagen attachment did not take place because platelets were subsequently washed out by the blood flow.
The three antiplatelet drug regimens affected coagulation as well, as fibrin deposition was significantly reduced. In a previous comparable study, clopidogrel significantly inhibited fibrin deposition on collagen, notably at low shear rate.14 The apparent anticoagulant effect provided by antiplatelet regimens may be the direct consequence of a reduction in platelet deposition, as fibrin deposition on collagen substrate occurs generally subsequent to platelet thrombus formation.27 It is also possible that this finding is a result of reduced platelet activation because activated platelets amplify the coagulation cascade by binding activated coagulation factors to form the tenase and prothrombinase complexes.28
Mechanisms involved in thrombus formation elicited by TF substrate are totally different from those on collagen. In contrast to collagen, TF is a procoagulant surface where thombi are composed of both fibrin and platelets with the latter deposited on top of the fibrin mesh.6 In addition, platelet activation and thrombin formation occurring on this surface are very high, as indicated by the plasma levels of βTG, T-AT, and FPA, which are 4, 42, and 55 times higher than with collagen, respectively (Tables 4 and 5). Moreover, on TF, thrombin is a prime mediator involved in thrombus formation, as indicated by a previous study using a selective inhibitor of factor Xa, the recombinant tick anticoagulant peptide.29 We found that neither antiplatelet regimen inhibited the thrombotic process on TF, indicating thereby that ADP and thromboxane A2 were not primarily involved in TF-induced thrombogenesis. It is possible that, on TF, the platelet inhibitor effect of aspirin and ticlopidine may have been overcome by high thrombin concentrations. The effect of the antiplatelet regimen on thrombus formation induced by surfaces coated with different levels of TF, which would generate different levels of thrombin, remains therefore to be determined.
We compared the efficacy of different antiplatelet and anticoagulant therapies in man using this experimental model. Criticisms with respect to its significance and clinical relevance may be raised. Whereas TF and types I and/or III collagens are important determinants of the thrombogenicity of ruptured human atherosclerotic lesions,9,10 the respective amount and the respective role of each of these substrates may be highly variable depending on the stage and depth of the lesion. Stent implantation results in blood exposure to a mixture of thrombogenic materials including collagen, TF, and foreign material. In our study, thrombus formation was only determined on a collagen- or a TF-coated surface. But, as discussed above, our thrombosis model has previously been shown to be useful in evaluating different antithrombotic agents.8,13,14 28-30One can note that results obtained with aspirin and ticlopidine on collagen-coated surfaces appear consistent with clinical data, ie, these drugs used alone moderately prevent arterial thrombus formation.
It is important to note that we examined the effect of antithrombotic drugs on early acute platelet thrombus formation, as perfusion times lasted only 3 minutes. This perfusion time was chosen because platelet deposition and thrombus formation in this model are maximum at 3 minutes.8 In addition, studies of antithrombotic drug effects on very early events of thrombus formation are important, as these events have profound impact on later ones.31,32Longer perfusion times give additional information on thrombus growth and thrombus stabilization. For example, whereas thrombin is not involved in initial thrombus formation on collagen, it plays a major role in thrombus growth and thrombus stabilization.33 Thus, whereas our experiments show that ADP and thromboxane A2 do not play a major role in mediating initial thrombin-driven platelet thrombus formation, whether these agonists play a role in thrombus stabilization is unknown. The late antithrombotic effects of aspirin, ticlopidine alone, or the combined aspirin + ticlopidine therapy remain to be determined.
In the present study, aspirin and ticlopidine were administered for 3 and 8 days, respectively. It is possible that the antithrombotic effect observed in volunteers treated by aspirin + ticlopidine may be present earlier than day 8. We chose these times of administration with regard to ticlopidine, which is known for its delayed onset of action.34 Thus, pharmacologic studies have indicated that significant inhibition of ADP-induced platelet aggregation occurs only after 3 to 5 days of oral administration of 250 mg twice daily of ticlopidine.34,35 However, it is not known whether the combination of aspirin + ticlopidine may shorten this delay of action. In a recent clinical study, the beneficial effect of the combined aspirin + ticlopidine therapy on thrombotic stent occlusions was observed within 3 days after stenting.3 Therefore, because the main clinical goal of antithrombotic therapy, notably in patients undergoing coronary stent implantation, is to be effective as soon as possible to prevent early stent thrombosis, a time-course of the antithrombotic effect of the combined aspirin + ticlopidine therapy is warranted.
Finally, ticlopidine has side effects, especially a risk of neutropenia. A new thienopyridine derivative, chemically related to ticlopidine, clopidogrel, has been recently developed. A large recent study showed that this product was quite safe.36 Whether the addition of clopidogrel to aspirin yields equivalent antithrombotic effects as ticlopidine has not been established. However, our study shows that ADP and thromboxane A2 have important and complementary roles in mediating acute arterial platelet thrombus formation and therefore that the combined ADP and thromboxane A2 antagonism is a promising therapeutic approach.
ACKNOWLEDGMENT
The authors thank S. Claudel for her expert assistance in the statistical analysis.
Address reprint requests to Yves Cadroy, MD, PhD, Laboratoire de Recherche sur l’Hémostase et la Thrombose, Pavillon Lefèbvre, CHU Purpan, 31059 Toulouse CEDEX, France; e-mail: cadroy.y@chu-toulouse.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.