Abstract
Little is known about the presence, frequency, and in vivo proliferative potential of stromal cells within blood-derived hematopoietic transplants. In this study, nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice were injected with human CD34+ peripheral blood cells (PBCs) or cord blood cells (CBCs, either enriched for CD34 or density-gradient separated mononuclear cells). Flow cytometric analysis 5 to 11 weeks after transplantation revealed the presence of a human lymphomyeloid hematopoiesis within the murine bone marrow. Immunohistochemical staining of bone marrow cell suspensions using human-specific antibodies showed human cells staining positive for human fibroblast markers, human von Willebrand factor (vWF) and human KDR (vascular endothelial growth factor receptor-2) in mice transplanted with CD34+ PBCs or CBCs, with mean frequencies between 0.6% and 2.4%. In stromal layers of bone marrow cultures established from the mice, immunohistochemical staining using human-specific antibodies revealed flattened reticular cells or spindle-shaped cells staining positive with human-specific antifibroblast antibodies (mean frequency, 2.2%). Cell populations of more rounded cells stained positive with human-specific antibodies recognizing CD34 (1.5%), vWF (2.2%), and KDR (1.6%). Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis and subsequent complementary DNA sequencing detected transcripts of human KDR (endothelial specific) and human proline hydroxylase-α (fibroblast specific) within the bone marrow and spleen of transplanted mice. Analysis of nontransplanted control mice yielded negative results in immunocytochemistry and RT-PCR. Cells expressing endothelial and fibroblast markers were also detected in the grafts before transplantation, and their numbers increased up to 3 log in vivo after transplantation. These results indicate that stromal progenitor cells are present in human cytokine-mobilized peripheral blood or cord blood that engraft in NOD/SCID mice.
Introduction
Bone marrow transplantation has provided substantial therapeutic benefit for patients suffering from hematologic diseases, such as leukemia and lymphoma. Moreover, in recent years, adult peripheral blood progenitor cells (PBCs) and cord blood cells (CBCs) have been introduced as alternative sources for hematopoietic progenitor cells in human transplants.1,2 Within the bone marrow, a close association between hematopoietic cells and stromal cells determines the survival and proliferation of hematopoietic progenitor cells, demonstrated in long-term bone marrow cultures.3-5 This culture technique has therefore been developed to detect and quantify primitive hematopoietic cell populations present within human hematopoietic transplants of various sources.6-8
More recently, studies using nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice have demonstrated that this murine xenotransplant model can be used efficiently to assess the transplantation potential of human hematopoietic stem cells derived from bone marrow, mobilized peripheral blood, or cord blood.9-13 As few as 5000 highly enriched CD34+CD38− cells from human cord blood could repopulate the bone marrow of recipient mice, with human hematopoietic stem cell progeny detectable within the murine bone marrow and circulation.10 The chimeric hematopoiesis established in NOD/SCID mice after transplantation of human adult bone marrow or PBCs was limited and had a maximum at about 6 to 8 weeks after transplantation, whereas transplantation of CBCs resulted in a more sustained production of human hematopoietic cells lasting for 10 to 12 weeks or longer.12 In addition, intravenous administration of different populations of human acute myelogenous leukemia cells served to characterize a hierarchy within the leukemic blasts with phenotypic separation of colony-forming progenitors from in vivo-repopulating and, therefore, leukemia-initiating cells.14 15
We have previously studied the engraftment of human progenitors from peripheral blood in SCID and NOD/SCID mice that had received cotransplants of rat fibroblasts transfected with the human interleukin-3 (IL-3) gene.16 17 However, the capability of human blood to contribute to the development of nonhematopoietic cells in NOD/SCID mice is as yet poorly characterized. Here, we describe the presence of cells within human blood that are able to form nonhematopoietic cells after transplantation into NOD/SCID mice. Bone marrow cultures have been established from the transplanted mice, and bone marrow cells have been analyzed directly via immunocytochemistry and reverse transcriptase-polymerase chain reaction (RT-PCR) to characterize the development of human fibroblasts and endothelial cells after transplantation of PBCs or CBCs.
Patients, materials, and methods
Patients and cells
After informed consent, patients with a diagnosis of a solid tumor or a lymphoid neoplasia underwent PBC mobilization by combination chemotherapy and granulocyte colony-stimulating factor (G-CSF). The PBCs were apheresed from whole blood after anticoagulation with acid-dextrose citrate. Mononuclear cells were separated from aliquots left over from quality control samples by Ficoll/Hypaque sedimentation (density, 1.077g/mL; Pharmacia, Freiburg, Germany) and incubated with a biotinylated antihuman CD34 antibody. CD34+ PBCs were separated using the Ceprate LC immunoaffinity column (CellPro, Bothell, WA) or immunomagnetic separation (Miltenyi Biotech, Bergisch Gladbach, Germany) according to the manufacturer's instructions. The purity of the CD34+PBC preparations was assessed by flow cytometry using the phycoerythrin (PE)-conjugated antihuman CD34 antibody HPCA-2 (Becton Dickinson, Heidelberg, Germany) and was found to be 73% to 98%. For some experiments, CD34+ cells were sorted by flow cytometry to a purity of more than 98% using a MoFlo cell sorter (Cytomation, Fort Collins, CO). Subsequent to isolation, CD34-enriched PBCs were suspended in column elution buffer containing phosphate-buffered saline (PBS) and 1% bovine serum albumin at 2 to 6 × 106cells/mL and were used for the experiments within 6 hours. Cord blood was sampled after informed consent was obtained from the mothers. The light-density (> 1.077 g/mL) fraction of CBCs was obtained after gradient separation of freshly collected blood samples using Ficoll/Hypaque solution and resuspended in RPMI medium containing 10% fetal calf serum (FCS; Cytogen, Berlin, Germany). CBCs were CD34+ enriched analogously to PBCs as described above, to a purity of 50% to 71%.
Mice
Breeding pairs of NOD/LtSz-SCID/SCID mice (originally obtained from Dr Leonard Schultz, Jackson Laboratories, Bar Harbor, ME) were expanded and maintained under pathogen-free conditions in the animal facility of the Max Delbrück Center of Molecular Medicine (Berlin, Germany). They were fed autoclaved standard diet purchased from Sniff (Soest, Germany) and acidified drinking water ad libitum. Mice were irradiated sublethally at 6 to 8 weeks of age with a dose of 250 to 300 cGy of a 137Cs-gamma source and transplanted with human cells within 3 to 5 hours thereafter. For transplantation, 0.2-mL samples of CD34+ PBCs (0.5-4 × 106), CD34+ CBCs (1.0-5 × 105), or mononuclear CBCs (0.8-1.2 × 107) were injected into the lateral tail vein. Mice with PBC grafts were cotransplanted with a stably transfected rat fibroblast cell line and expressing the humanIL-3 gene (Rat-hIL-3) as described previously.16 Briefly, transfected cells were propagated in Dulbecco modified Eagle medium (DMEM, Gibco, BRL, Paisley, United Kingdom) with 10% FCS. Trypsinized cells were washed, resuspended in ice-cold culture medium without additives, and mixed with matrigel (Basement Membrane Matrix 40234; Becton Dickinson, Bedford, MA) as described.17 Five million cells were injected subcutaneously into the lateral abdomen.
Cell preparation, staining, and flow cytometric analysis
Mice were killed by cervical dislocation 5 to 11 weeks after transplantation. Their blood and bone marrow cells were collected for analysis. Single-cell suspensions were prepared, cell counts were performed, and viability was determined by trypan blue exclusion. Following blocking of Fc receptors by pretreatment of the cells with human serum and an antimouse IgG receptor antibody (clone 2.4G2, Pharmingen, San Diego, CA), cell staining was carried out as described.17 18 Human- or mouse-specific monoclonal antibodies conjugated with either fluorescein isothyocyanate (FITC) or PE were used in combinations to identify cells of human and murine origin: mouse antihuman CD45 and antihuman HLA class-I (Pharmingen); anti-CD1a, -CD2, -CD7, -CD10, -CD13, -CD19, -CD33, -CD56, and anti-HLA-DR (Immunotech, Marseille, France); anti-CD34 and -CD38 (Becton Dickinson); and rat-antimouse-CD45 (Pharmingen). In each experiment, cells from a NOD/SCID mouse not transplanted with human cells were stained with the same antibodies as a control. Background fluorescence was assessed by including isotype-matched antibodies conjugated with FITC or PE. Cell analysis was performed with a FACSCalibur system (Becton Dickinson) using CellQuest software. Each measurement contained 10 000 events. Dead cells were excluded by outgating of propidium iodide-stained cells.
Immunohistochemistry and chimeric bone marrow cultures
Bone marrow cells were prepared from the transplanted animals 5 to 11 weeks after injection of the human cells and from untreated control animals, transferred onto poly-l-lysin-precoated glass slides (Marienfeld, Bad Mergentheim, Germany) and fixed with glutaraldehyde, or seeded at 1 to 2 × 106/mL into slide flasks (NUNC, Wiesbaden, Germany) using a culture volume of 3.0 mL in Dexter-type human long-term bone marrow culture medium consisting of 12.5% FCS (PAN Systems, Passau, Germany), 12.5% horse serum (Sigma, Munich, Germany), 5 × 107 mol/L hydrocortisone (Sigma) in Iscove modified Dulbecco medium (IMDM, Gibco, RL) at an osmolarity of 340 mosm/L. Cultures were supplemented with 10% conditioned medium of the Rat-hIL-3 line, which produces 10 to 15 ng hIL-3/106 cells per 24 hours constitutively as measured with a human-specific IL-3 enzyme-linked immunosorbent assay (ELISA)17 and were incubated at 33°C in a humidified atmosphere with 5% CO2. Two thirds of the medium was exchanged every 2 to 3 days and nonadherent cells were removed. The cultured stromal cells were examined by light microscopy for monolayer formation and morphology. The cultures were terminated 2 to 3 weeks after seeding by removal of the culture medium, 3 times flushing with PBS, and fixation in 0.04% glutaraldehyde solution. Before fixation, some cultures were stimulated with recombinant human 2 ng/mL vascular endothelial growth factor (VEGF)19 for 5 to 8 hours at 37°C. Blocking was performed with a buffer containing 0.2% bovine serum albumin.
Both the bone marrow cells fixed directly on glass slides and the bone marrow culture stromal layers were incubated with human-specific antibodies directed to human CD45 (clone HI30), human CD1a (clone CBT6), human CD14 (clone UCHM1), human CD33 (clone WM 53), all purchased from Dianova GmbH, Hamburg, Germany; human CD34 (clone HPCA-2) and human CD38 (clone HB-7) from Becton Dickinson; human CD31 (PECAM-1, clone EN-4 from Monosan [Sanbio], Uden, The Netherlands) and human CD106 (vascular cell adhesion molecule [VCAM]-1, clone 51-10C9) from Pharmingen; human HLA class-I (clone W6/32), human CD68 (clone PG-M1), and human von Willebrand factor (vWF; clone F8/86) from DAKO Diagnostics GmbH (Glostrup, Denmark). Two different monoclonal antibodies directed to membrane antigens expressed on human fibroblasts (clone AS02 from Dianova, Hamburg, Germany, clone 5B5 recognizing the α subunit of proline hydroxylase, which is specifically expressed in fibroblasts20 from DAKO) were used to stain human fibroblastic stromal cells. Antibody against KDR was used as described.21 The antibody-labeled cells were stained using the LSAB-2 immunoperoxidase staining kit (DAKO). Nuclear counterstaining was performed using hematoxylin (Merck, Darmstadt, Germany). All staining included negative controls analogously processed using cells from nontransplanted animals, which were negative for the different antibodies used. All staining contained 1 to 3 fields with isotype-matched, nonbinding control antibodies that did not bind to any cells on the slide. Slides were screened for the presence of human cells by light microscopy. In representative fields, 400 individual cells were counted to determine the percentage of positive cells. Selected slides, especially areas with high densities of positive-staining cells, were also documented by microphotography.
Reverse transcriptase-polymerase chain reaction analysis
RNA from mouse bone marrow cell suspensions was extracted using the guanidinium thiocyanate protocol.22 Reverse transcription was performed from 1 to 2 μg of RNA using a oligo-dT primers and reverse transcriptase (Perkin Elmer, Norwalk, CT). The presence of human-specific RNA within the bone marrow of transplanted mice was confirmed by PCR using primers complementary to human KDR, with 40 cycles of 98°C (2 seconds), 65°C (1 minute), and 72°C (2 minutes), and subsequently the nested primers sense 5′-GTGTAACCCGGAGTGACCAAGGAT-3′ and antisense 5′-GATGTGATGCGGGGGAGGAA-3′ with 35 cycles of 98°C (2 seconds), 57.6°C (1 minute), and 72°C (2 minutes). Detection of transcripts for human proline hydroxylase was done using the primers, sense, 5′-GATGGGGCAGGGGATGTTGACGAC-3′ and antisense, 5′-GCGGGAGGGACG CAGCGAGACT-3′ with 35 cycles of 95°C (2 minutes), 62.5°C (1 minute), and 72°C (2 minutes). Control reactions omitting the reverse transcription step were included and yielded negative results. PCR products were electrophoresed on 2% agarose gels stained with ethidium bromide. PCR product sizes were at 790 bp for KDR (first PCR), 482 bp for KDR (second PCR), and 429 bp for proline hydroxylase. The sensitivity of the RT-PCR analysis was 0.3% for human proline-4-hydroxylase and 1% to 3% for human KDR as determined in dilution experiments with RNA isolated from fibroblasts or human umbilical vein endothelial cells, respectively. For both PCRs, end products amplified from RNA derived from bone marrow of transplanted animals were cloned into a pCR vector and sequenced using an ABI Prism 377 sequencer. Identical sequences as found in the database for the human KDR and proline hydroxylase genes were obtained. As an additional control, RNA was isolated from macrophages derived from 10-week-old human Dexter-type long-term bone marrow cultures established from bone marrow of patients with lymphoma. Macrophages were labeled with anti-CD45 FITC and anti-CD14-PE antibodies and the CD45+CD14+ fraction was sorted by flow cytometry on a MoFlo cell sorter (Cytomation). After pretreatment with VEGF as described above, RNA was isolated and RT-PCR for KDR was performed, showing a negative result. The presence of human-specific DNA was confirmed by amplification of sequences of a 850-bp DNA fragment of the α-satellite DNA of human chromosome 17.24 Genomic DNA was extracted through a QIAmp tissue kit (Qiagen, Hilden, Germany). Briefly, the DNA of lysed cells was adsorbed to a silica matrix, washed, and eluted with QuiaAmp elution buffer by centrifugation. Amplification of the centromere-specific human fragments of chromosome 17 was performed using primers corresponding to the primer pair 17a1/17a2 as described.25 The primers were elongated to 25 nucleotides each for use at a higher annealing temperature. The 5′ primer (5′-GGGATAATTTCAGCTGACTAAACAG-3′) covers the positions 15 to 39 and the 3′ primer (5′-TTCCGTTTAGTTAGGTGCAGTTATC-3′) covers the positions 867 to 891 of the sequence HSSATA17 (GenBank No.M13882). The PCR reaction contained 200 μmol/L each of the respective nucleotides, 250 nmol/L of each primer, 2 mmol/L MgCl2, and 250 ng of genomic DNA. Thirty-five cycles were run at 94°C (1 minute), 60°C (1 minute), 72°C (10 minutes) with a final elongation step at 72°C for 10 minutes. Amplified DNA fragments were electrophoresed through 1.75% agarose gels and stained with ethidium bromide. Genomic DNA from human breast carcinoma (cell line MaCa 3366) was used as a positive control and liver tissue from a nontransplanted NOD/SCID mouse served as a negative control.
Results
Human hematopoietic cell engraftment in NOD/SCID mice
Transplants of all 3 sources, CD34+ PBCs, light-density CBCs, or CD34+ CBCs, resulted in high levels of human hematopoietic cells in NOD/SCID mice. After CD34+PBCs, up to 44%, and after mononuclear CBCs or CD34+ CBCs, up to 40% or 72%, respectively, of human CD45+ cells were detected in the bone marrow of the animals (Table1). Flow cytometry demonstrated the development of human cells with both lymphoid and myeloid phenotypes (Figure 1, Table 1). PCR analysis of genomic DNA isolated from the bone marrow of engrafted mice confirmed the presence of human cells (not shown). Thus, high levels of human hematopoietic engraftment were obtained with human CBCs or adult PBCs.
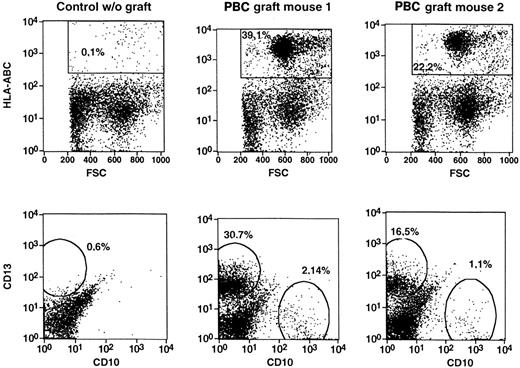
Human progenitor cells from peripheral and cord blood initiate a lymphomyelopoiesis in NOD/SCID mice.
Single-cell suspensions were prepared from bone marrow of NOD/SCID mice transplanted with CD34-enriched PBCs or light-density CBCs, incubated with fluorescence-tagged monoclonal antibodies directed to human cell surface antigens, and analyzed by flow cytometry. Dot-plot analyses of dual marker-labeled cell preparations were performed. The percentages given in the panels represent the percentage of cells in the total ungated population (10 000 events). Representative analyses of the experiments in Table 1 are shown.
Human progenitor cells from peripheral and cord blood initiate a lymphomyelopoiesis in NOD/SCID mice.
Single-cell suspensions were prepared from bone marrow of NOD/SCID mice transplanted with CD34-enriched PBCs or light-density CBCs, incubated with fluorescence-tagged monoclonal antibodies directed to human cell surface antigens, and analyzed by flow cytometry. Dot-plot analyses of dual marker-labeled cell preparations were performed. The percentages given in the panels represent the percentage of cells in the total ungated population (10 000 events). Representative analyses of the experiments in Table 1 are shown.
Presence of human stromal cells in the bone marrow of transplanted mice
Bone marrow cell suspensions were analyzed immunocytochemically from transplanted mice. Relatively comparable levels of cells expressing human HLA-I, CD34, CD33, CD38, or CD14/CD68 as found in the flow cytometric analysis were detected (Table2). In all 3 groups, that is, mice that had received CD34+ PBCs, light-density CBCs, or CD34+ CBCs, engraftment of hematopoietic cells was accompanied by engraftment of cells expressing fibroblast and endothelial cell markers, as detected by staining with antibodies to human KDR, human vWF, or the human fibroblast-specific antibodies AS02 or 5B5 (Table 2). Stromal marker-positive cells engrafted at significantly lower levels compared with hematopoietic cells but were detected at similar frequencies after transplantation with all 3 transplant cell populations (Figure 2H,I and Table 2). Cells expressing human fibroblast markers were detected in the majority of the mice, with mean frequencies of 0.8% in PBC-transplanted mice and up to 3.6% in CBC-transplanted mice. Endothelial cells were detected by staining with antihuman vWF or anti-KDR antibodies (mean frequency, 0.4%-0.9%). Bone marrow cell preparations from nontransplanted animals were included in all experiments and did not stain with any of the antibodies used, indicating that the positive cells were of donor origin (Figure 2). A human-specific RT-PCR of RNA from the chimeric bone marrow cell suspensions was established to detect the presence of transcripts of 2 human genes encoding proteins detected with the above-described antibodies, namely, human KDR and the α subunit of human proline hydroxylase20 recognized by the 5B5 human fibroblast-specific antibody (Figure 3). The transcripts were only seen in mice transplanted with human cells, but not in nontransplanted control mice. Only 3 of 9 mice were positive for KDR, which is in line with the relatively high detection limit of the KDR PCR of 1% to 3% (see “Patients, materials, and methods”) and the lower mean frequency found in immunohistochemistry (Table 2). Sequencing of PCR products showed identity of the obtained sequences with the human complementary DNAs (cDNAs) obtained from the database. Amplification of DNA was excluded by separate analyses omitting the reverse transcription step, which proved negative (not shown). An additional control was performed with RNA from sorted human macrophages (CD45+CD14+) derived from human Dexter-type long-term bone marrow cultures, which was negative, indicating that human macrophages were not the source of the signal. These data indicate the presence of human nonhematopoietic (stromal) cells after transplantation of human blood into NOD/SCID mice.
Immunostaining of stromal marker-positive cells.
Immunostaining is shown for human-specific cell surface markers in stromal layers of chimeric bone marrow cultures (A-G) or cell suspensions (H,I,K) from NOD/SCID mouse bone marrow harvested 5 to 11 weeks after transplantation with human blood cells. Mice were transplanted with human CD34-enriched PBCs (A,B,D,H,I,J) or light-density CBCs (C,D,G). The antibodies used were directed to the following human cell surface antigens: (A) HLA class-I, (B) 5B5 (fibroblast), (C) vWF, (D,E) KDR/flk-1, (F) HLA class-I, (G) CD34, (H) HLA class-I, (I) AS02 (fibroblast), (K) HLA class-I. Positive staining appears as red or brown. Controls from bone marrow from nontransplanted mice were processed in parallel for all markers (F,K). (Original magnification, 40-fold [A], 400-fold [B-G], 1000-fold [H-K].)
Immunostaining of stromal marker-positive cells.
Immunostaining is shown for human-specific cell surface markers in stromal layers of chimeric bone marrow cultures (A-G) or cell suspensions (H,I,K) from NOD/SCID mouse bone marrow harvested 5 to 11 weeks after transplantation with human blood cells. Mice were transplanted with human CD34-enriched PBCs (A,B,D,H,I,J) or light-density CBCs (C,D,G). The antibodies used were directed to the following human cell surface antigens: (A) HLA class-I, (B) 5B5 (fibroblast), (C) vWF, (D,E) KDR/flk-1, (F) HLA class-I, (G) CD34, (H) HLA class-I, (I) AS02 (fibroblast), (K) HLA class-I. Positive staining appears as red or brown. Controls from bone marrow from nontransplanted mice were processed in parallel for all markers (F,K). (Original magnification, 40-fold [A], 400-fold [B-G], 1000-fold [H-K].)
RT-PCR amplification of RNA from mice.
RNA was isolated from the bone marrow or spleen cell suspensions of transplanted or nontransplanted mice as indicated. The cDNA was prepared and analyzed for the presence of transcripts for human KDR or human proline hydroxylase as described in “Patients, materials, and methods.” The PCR products were separated on agarose gels and visualized with ethidium bromide. Negative control, mock PCR using water instead of cDNA; positive control, cDNA from human umbilical vein endothelial cells (HUVECs) for KDR, or KMST-6 fibroblasts of human Dexter cultures for proline hydroxylase. CD45+CD14+: analysis of sorted cells from Dexter cultures as described in “Patients, materials, and methods.” A total of 3 of 9 transplanted mice were positive for human KDR cDNA and 6 of 8 mice for proline hydroxylase. BMC indicates analysis from a bone marrow culture established from the bone marrow of a mouse transplanted with CD34+ PBCs.
RT-PCR amplification of RNA from mice.
RNA was isolated from the bone marrow or spleen cell suspensions of transplanted or nontransplanted mice as indicated. The cDNA was prepared and analyzed for the presence of transcripts for human KDR or human proline hydroxylase as described in “Patients, materials, and methods.” The PCR products were separated on agarose gels and visualized with ethidium bromide. Negative control, mock PCR using water instead of cDNA; positive control, cDNA from human umbilical vein endothelial cells (HUVECs) for KDR, or KMST-6 fibroblasts of human Dexter cultures for proline hydroxylase. CD45+CD14+: analysis of sorted cells from Dexter cultures as described in “Patients, materials, and methods.” A total of 3 of 9 transplanted mice were positive for human KDR cDNA and 6 of 8 mice for proline hydroxylase. BMC indicates analysis from a bone marrow culture established from the bone marrow of a mouse transplanted with CD34+ PBCs.
Detection of human nonhematopoietic cells in bone marrow cultures from engrafted NOD/SCID mice
To further characterize the stromal cell nature of the detected cell populations, bone marrow cultures were established. To stimulate the proliferation of human hematopoietic cells, conditioned medium of the Rat-hIL-3 cell line was added. The cultures showed formation of adherent cell islands within 1 week after seeding, which expanded and formed semiconfluent stromal layers within 2 to 3 weeks. Cobblestone areas formed underneath the stroma and became more prominent by week 3. Immunohistochemical analysis of the stromal layers revealed the presence of hematopoietic cells staining positive with human-specific antibodies against HLA class-I, CD34, CD33, CD38, CD14, CD68, and CD1a antigens, indicating the proliferation and differentiation of immature myeloid as well as monocyte-macrophage and dendritic cell populations of human origin within this environment (Table3). Immunostaining of the cultures with human anti-HLA class-I antibody revealed, in addition to hematopoietic cells, the presence of human HLA class-I+ cells with branched or spindle-shaped morphology (Figure 2A). These were often found in close association with smaller and round human HLA class-I+ cells displaying the morphology of hematopoietic clusters. Relatively large cells exhibiting a flattened reticular or dendritic morphology stained positive for VCAM-1 (CD106, not shown) and antigens specifically expressed in human fibroblasts (Figure2B).
Cells expressing human vWF, the human VEGF-type 2 receptor KDR, and human CD34 were also seen and displayed either a spindle-shaped or a more rounded morphology with small dense nuclei (Figure 2C-E,G). In some cases, clusters with about 30 to 100 cells staining positive for endothelial markers were observed (Figure 2C,E), whereas in many other cultures, these cells were distributed more evenly. On average, these cells were found at frequencies of about 1.5% to 2% within the cultures (Table 3). Control staining using isotype-matched nonbinding antibodies, or staining of analogous preparations from nontransplanted control mice with the stromal antibodies, were included in all experiments and proved negative. Spindle-shaped adherent cells expressing CD34 were repeatedly seen in the direct vicinity of small round CD34+ (most likely hematopoietic) cells and adhered to them (Figure 2G). The human stromal cells retained their proliferative potential on passage of the bone marrow cultures through 2 more subcultivation steps.
Proliferation of stromal cells in NOD/SCID mice
Immunocytochemical analyses from CD34+ CBC before transplantation revealed the presence of human KDR−, vWF−, and CD31+ as well as human fibroblast marker-positive cells with a similar, relatively low frequency as in the bone marrow of the transplanted mice (Table4). Calculations of the numbers of transplanted stromal cells and the recovered total stromal cell numbers in the mice after transplantation were performed assuming that one femur contains 8% of the entire mouse bone marrow. An up to 3 log amplification in the numbers of the fibroblastic and endothelial marker-positive cells was seen in the transplanted mice, however, depending on the type of transplanted cells, with highest expansion factors after transplantation of CD34+ CBCs, and lowest factors when using unseparated CBCs (Table 4). It is unclear at this time which cell (sub)populations are the origin of the stromal cells seen after transplantation, and if the potential stromal precursor cells already carry stromal differentiation markers.
Taken together, these data indicate that transplantation of human mobilized PBCs or CBCs results in engraftment of not only hematopoietic but also of stromal cells.
Discussion
In this study, engraftment of both human hematopoietic and human stromal cells could be visualized in xenotransplanted NOD/SCID mice. The presence of human endothelial and fibroblastic cells was analyzed in chimeric bone marrow and in cultures established from the marrow samples by expression of human tissue-specific markers. Our results indicate the presence of donor cells with both endothelial cell and fibroblastic developmental potential in NOD/SCID mice after transplantation with CBCs or cytokine-mobilized adult PBCs.
Previous work has demonstrated that human hematopoietic cells injected into sublethally irradiated NOD/SCID mice can initiate subsequent lymphomyeloid reconstitution, as shown by cell surface marker analysis of human hematopoietic cells present within the murine bone marrow and circulation: Larochelle and coworkers10 and Wang and colleagues26 found that a discrete population of SCID mouse-repopulating cells (S-RCs) leads to long-term repopulation of the NOD/SCID bone marrow with human hematopoietic cells. The reported incidence of S-RCs in mobilized peripheral blood is about 1 in 6 to 8 × 106 mononuclear cells.26 Assuming that most S-RCs were CD34+, and that the mobilized PBC grafts used by Wang and associates might have contained 1% to 2% of CD34+ cells, about 10 to 20 S-RCs were injected into each of our animals. Similarly, transplantation of 107light-density CBCs per animal corresponding to about 10 S-RCs, as calculated according to Wang and coworkers,26resulted in a hematopoietic engraftment in our mice that is comparable to previously published data.10,14 In contrast to the mice transplanted with CD34+ selected PBCs bearing Rat-hIL-3 cotransplants, which exhibited predominantly monomyelocytic engraftment, the majority of human cells generated in mice grafted with CBCs belonged to the B-lymphoid lineage. T lymphopoiesis was practically absent in recipients of either transplant type, which is in accordance with previous results.13,16 17
The relatively high numbers of human cells seen in mice transplanted with mobilized CD34+ PBCs may at least in part be due to the continuously high endogenous levels of human IL-3 maintained by the Rat-hIL-3 cotransplants. In earlier studies, subcutaneous transplants of matrigel-clotted Rat-hIL-3 cells applied to young SCID mice produced high levels of circulating human IL-3 (mean 68 ng/mL in mouse serum) for at least 10 to 12 weeks after transplantation.16 A continuous endogenous supply of this cytokine may be superior to repeated administrations of exogenous recombinant growth factors applied via subcutaneous or intraperitoneal routes.27 Mice receiving CBC grafts were not cotransplanted with Rat-hIL-3 cells. In contrast to adult PBCs, cord blood mononuclear cells have previously been shown in vitro to produce granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-3 and to undergo proliferation even in the absence of added growth factors.28 This is in agreement with our own observations from comparative experiments in NOD/SCID mice transplanted with CD34+ CBCs, which showed that mice that received Rat-IL-3 cotransplants developed higher levels of human hematopoiesis than mice that were cotransplanted with the untransfected parent cell line. However, numbers of stromal cells in the transplanted mice were not affected (manuscript in preparation).
So far, few data are available that describe the presence of human nonhematopoietic cells after transplantation of human blood into NOD/SCID mice. Fernandez and coworkers29 characterized fibroblast progenitors within human mobilized peripheral blood using the colony-forming unit-fibroblast (CFU-F) assay. The PBC population giving rise to fibroblastic colonies could be enriched using antibodies against mesenchymal cells; however, expression of CD34 was not determined. Using such antibodies, mesenchymal progenitors have been isolated from bone marrow, which have fibroblastic, osteogenic, chondrogenic, myogenic, ligament-type, and adipogenic differentiation potential in vitro.30-34 On transplantation of cultured mesenchymal progenitors, an osteogenic differentiation potential was confirmed in vivo.35,36 However, mesenchymal progenitors have so far not been reported to be mobilized from bone marrow to blood or to circulate. Almeida-Porada and colleagues37 transplanted human bone marrow- or peripheral blood-derived CD34+ populations into preimmune fetal sheep. In about 40% of animals they could find human stromal cells in long-term bone marrow cultures established from the chimeric bone marrow. Interestingly, some animals showed donor stromal cell engraftment in the absence of any detectable hematopoietic engraftment, indicating that hematopoietic and stromal cell precursors contained within the grafted human cells may be derived from different progenitors. A donor origin of fibroblastic stromal cells has been found in a subgroup of patients with chronic myelogenous leukemia after allogeneic stem cell transplantation,38 which is in line with our results. A study by Simmons and colleagues39 in CD34+ bone marrow cells and the study of Almeida-Porada and coworkers37 found that stromal progenitor cells copurify with the fraction of CD34+ cells.
The CD34+ endothelial cell progenitors have been successfully cultured and differentiated in vitro from CD34-enriched peripheral blood cells by Asahara and associates.40 These cells were able to form capillary tubes and endothelial sprouts on fibronectin-coated plastic and incorporated into regenerating endothelial lesions in vivo as shown after transplantation into mouse and rabbit models. The cells also expressed CD31, KDR/flk-1, and vWF. It is likely that this cell population or its progeny also developed in our system. The circulation and mobilization of endothelial progenitor cells has been described in animals. Circulating endothelial cell progenitors have been found by Shi and coworkers23 in a canine bone marrow transplantation model. Takahashi and associates41 have demonstrated that pretreatment of mice with GM-CSF, similarly as ischemia, results in mobilization of CD34+ endothelial cell progenitors to the blood. A capacity of cord blood to initiate blood vessel formation in thymus has very recently been described in transplanted NOD/SCID mice by Crisa and colleagues.42 Lin and coworkers43 have recently shown that after allogeneic transplantation of human bone marrow, or in one case, peripheral blood, donor-type endothelial progenitors are found in the recipient patients. Implanted intraventricular cardiac devices can be colonized with endothelial progenitors in patients receiving transplants with endothelial progenitors.44 These findings are in line with our results and indicate that the transplantation of endothelial progenitor cells contained in hematopoietic stem cell grafts has a relevance for human clinical blood stem cell transplantation. Our data indicate that endothelial and also fibroblastic progenitors reside in the bone marrow after transplantation of human blood-derived CD34+cells and that they engraft relatively regularly in NOD/SCID mice.
Stromal cell populations as analyzed after transplantation were also present in the grafts before transplantation. However, these populations nominally amplified at much higher ratios in the mice after transplantation of CD34+ CBCs than after light-density CBCs. This suggests that the stromal cells found in the mice after transplantation may be generated from small amounts of precursor cells that are present within the CD34+ population. Further experiments using more defined cell populations will be necessary to determine which cell populations are giving rise to the stromal cell activity. Ziegler and colleagues45 have defined a small, KDR+ subfraction within human CD34+ cell populations as very primitive, pluripotent stem cells. It is likely that this cell population was also present in the CD34+-enriched and CBC grafts analyzed in this study. However, both the numbers of KDR+ cells found in the mice after transplantation, and the expression of cells staining positive for vWF, an endothelial maturation marker, make it unlikely that the detected endothelial cell population represents pluripotent CD34+KDR+ progenitors.
The analysis of stromal cell types after transplantation into NOD/SCID mice as described here should allow more systematic analyses also of other previously unrecognized cell populations contained in human hematopoietic transplants and their potential to form stromal cells of various lineages. Our results implicate that CD34-enriched PBC preparations or CBCs are a potential source for the therapeutic delivery of stromal cells with routine clinical transplants. It has been postulated that microenvironmental damage plays a role in the prolonged pancytopenia observed following application of myelotoxic drugs used for remission induction and consolidation in the treatment of hematologic malignancies.46-48 Our results indicate that stromal cells or their progenitors are cotransplanted in hematopoietic stem cell transplantation.
Thus, the NOD/SCID mouse model allows assessment of the frequency as well as the developmental potential of circulating nonhematopoietic cells and provides a basis to investigate potential associations between hematopoietic cell engraftment kinetics and stromal cell content in human hematopoietic grafts. Finally, the technique should provide a preclinical model to design clinical protocols involving the transplantation of defined stromal cell populations.
Acknowledgments
The excellent technical assistance of Elisete De Lima-Hahn and Monika Becker is gratefully acknowledged.
Supported by the Deutsche Forschungsgemeinschaft through grant Fi-527/1 (to I.F.) and SFB 364 (to R.H.), the Bundesministerium für Bildung und Forschung (07ITU04 to R.H.), and the José-Carreras Leukemia Foundation (to I.F. and R.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Reinhard Henschler, Institute of Transfusion Medicine and Immune Hematology, Blood Donation Service of the German Red Cross, Sandhofstrasse 1, D-60528 Frankfurt, Germany.

![Fig. 2. Immunostaining of stromal marker-positive cells. / Immunostaining is shown for human-specific cell surface markers in stromal layers of chimeric bone marrow cultures (A-G) or cell suspensions (H,I,K) from NOD/SCID mouse bone marrow harvested 5 to 11 weeks after transplantation with human blood cells. Mice were transplanted with human CD34-enriched PBCs (A,B,D,H,I,J) or light-density CBCs (C,D,G). The antibodies used were directed to the following human cell surface antigens: (A) HLA class-I, (B) 5B5 (fibroblast), (C) vWF, (D,E) KDR/flk-1, (F) HLA class-I, (G) CD34, (H) HLA class-I, (I) AS02 (fibroblast), (K) HLA class-I. Positive staining appears as red or brown. Controls from bone marrow from nontransplanted mice were processed in parallel for all markers (F,K). (Original magnification, 40-fold [A], 400-fold [B-G], 1000-fold [H-K].)](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/12/10.1182_blood.v96.12.3971/5/m_h82300426002.jpeg?Expires=1766131099&Signature=LrSarIBJRywmrjtDTVZj0kQMFSpdIEmqXcel80LIFBmrrnFudsQggu6G7D6EzorbnGlBHf2YSNMHi8k7JLoiRIsQWQWjoQS2baTrzeyuSdvjU2XW26M94dbgKUhpk2ORFkElzAZMleFuhcuTMNkoi94FyuLaCxnwYpqCVyUGRo2HcUEjQNtQGEu86Jg37k4Qy1m4-8bZO9ZvMU28mkn9F9HSM3UfTCsAf3HsTTvLA5PlvaKtmwRudKZIRe3gVvVw3Dpwl29id4tu1Sx-LL2Q5ZJNYhMoGswl-eaam2awGaIWeOHmv92~qAvGv-uHsvVUKfKwudINEEsOFODXfYD6hg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

