Abstract
Recent studies suggest that the Bcl-2 and mitogen-activated protein kinase (MAPK) pathways together confer an aggressive, apoptosis-resistant phenotype on acute myelogenous leukemia (AML) cells. In this study, we analyzed the effects of simultaneous inhibition of these 2 pathways. In AML cell lines with constitutively activated MAPK, MAPK kinase (MEK) blockade by PD184352 strikingly potentiated the apoptosis induced by the small-molecule Bcl-2 inhibitor HA14-1 or by Bcl-2 antisense oligonucleotides. Isobologram analysis confirmed the synergistic nature of this interaction. Moreover, MEK blockade overcame Bcl-2 overexpression-mediated resistance to the proapoptotic effects of HA14-1. Most importantly, simultaneous exposure to PD184352 significantly (P = .01) potentiated HA14-1–mediated inhibition of clonogenic growth in all primary AML samples tested. These findings show that the Bcl-2 and MAPK pathways are relevant molecular targets in AML and that their concurrent inhibition could be developed into a new therapeutic strategy for this disease.
Introduction
Leukemogenesis is a complex process involving multiple genetic alterations that result in the abnormal regulation of proliferation, differentiation, and apoptosis of leukemic blasts, leading to their accumulation.1 These aberrations also affect the sensitivity of leukemic cells to chemotherapeutic agents and, hence, the therapeutic outcome. Prior investigations by our group and others have established Bcl-2 as an important prognostic factor in acute myelogenous leukemia (AML)2,3 and have shown that inhibition of Bcl-2 expression induces apoptosis and sensitizes AML cells to chemotherapy.4,5 Recently, we have examined the role of the mitogen-activated protein kinase kinase (mitogen-activated protein kinase [MAPKK] or MAPK kinase [MEK])/extracellular signal-regulated kinase (ERK, hereafter referred to as MAPK) pathway, a key integration point in the signaling cascade regulated by growth factor receptors.6 We have demonstrated that constitutive MAPK activation is frequently found in primary AML samples (74% of the cases), that it promotes AML cell growth and survival without affecting Bcl-2 expression levels,7 and that it is independently prognostic for survival in patients with AML (S.M.K., manuscript submitted, September 2001). Albeit distinct, the Bcl-2 and MAPK pathways may crosstalk with each other,8-10 jointly contributing to leukemogenesis.11 Our recent evidence that constitutive MAPK activation and low, antiapoptotic Bax/Bcl-2 ratios together confer a uniformly poor prognosis on AML patients (S.M.K., manuscript submitted, September 2001) underscores the clinical relevance of such crosstalk between antiapoptotic and growth-promoting pathways and suggests that it could be exploited with therapeutic intent.
Study design
Cell cultures
AML cell lines (OCI-AML3, HL-60, and KG1) were cultured under standard conditions14 and harvested in log-phase growth for every experiment. Bone marrow samples were obtained during routine diagnostic assessment after informed consent, in accordance with regulations and protocols sanctioned by the Human Subjects Committee of the University of Texas M. D. Anderson Cancer Center. Cell viability was evaluated by triplicate counting of trypan blue dye–excluding cells under a light microscope. AML blast colony assays were performed as previously described.15 HL-60 cells stably transfected with either Bcl-2 (HL-60/Bcl-2) or empty vector control (HL-60/neo) were kindly provided by Dr K. Bhalla (Moffitt Cancer Center, University of South Florida, Tampa, FL).16PD184352 (2-[2-chloro-4-iodo-phenilamino]-N-cyclopropylmethoxy-3,4-difluoro-benzamide), a highly selective inhibitor of MEK activation,13,17 was kindly provided by Dr J. S. Sebolt-Leopold (Cancer Molecular Sciences, Pfizer Global Research & Development, Ann Arbor, MI). HA14-1 (ethyl 2-amino-6-bromo-4-[1-cyano-2-ethoxy-2-oxoethyl]-4H-chromene-3-carboxylate), a small organic molecule selected for its ability to bind the surface pocket of Bcl-2, thereby disrupting its heterodimerization with Bax12 (M.A., manuscript in preparation, December 2001), was obtained from Maybridge (Cornwall, United Kingdom); a structurally related compound lacking Bcl-2 binding activity (ethyl 4-[cyano(ethoxycarbonyl)methyl]-4H-chromene-3-carboxylate) was used as negative control in all the experiments involving HA14-1. P-ethoxy Bcl-2 AS, complementary to the Bcl-2 translation initiation site (5′-CAGCGTGCGCCATCCTTCCC-3′), previously shown to efficiently decrease Bcl-2 expression in myeloid leukemic cells, or a scrambled sequence (5′-TCGCCACTGGATCCTGCCCG-3′, nonsense, [NS]) were incorporated into liposomes as previously described.5
Western blotting and apoptosis assays
MAPK phosphorylation was detected by Western blot analysis as previously described.7 To measure mitochondrial membrane potential (ΔΨm), cells were loaded with CMXRos (300 nM) and MitoTracker Green (100 μM, both from Molecular Probes, Eugene, OR) for 1 hour at 37°C. The ΔΨm was then assessed by measuring CMXRos retention (red fluorescence) while simultaneously adjusting for mitochondrial mass (green fluorescence).18Caspase activation was detected by flow cytometry using a fluorescein-conjugated cell-permeable peptide (FAM-VAD-FMK) that irreversibly and selectively binds to activated caspases (caspase-1 through -9) (CaspaTag, Intergen, Purchase, NY). Annexin V binding of externalized phosphatidylserine, cell permeability, and staining of nuclear DNA were analyzed as previously published.7
Statistical analysis
Synergism, additive effects, and antagonism were assessed using the Chou-Talalay method19 and Calcusyn software (Biosoft, Ferguson, MO). Briefly, the dose-effect curve for each drug alone was determined based on the experimental observations using the median-effect principle; the combination index (CI) for each experimental combination was then calculated according to the following equation:
where (D)1 and (D)2 are the doses of drug 1 and drug 2 that have x effect when used in combination and (Dx)1 and (Dx)2 are the doses of drug 1 and drug 2 that have the same x effect when used alone. When CI = 1, this equation represents the conservation isobologram and indicates additive effects. CI values less than 1.0 indicate a more than expected additive effect (synergism).
Results and discussion
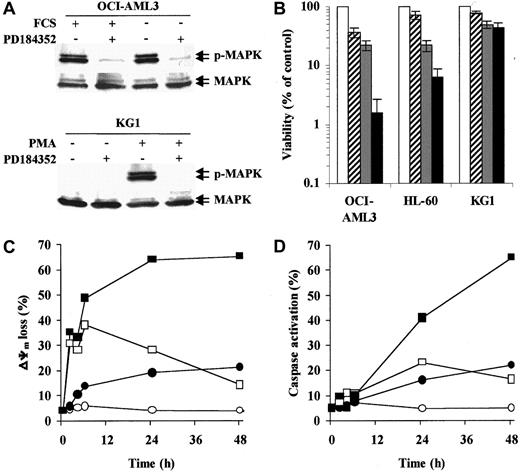
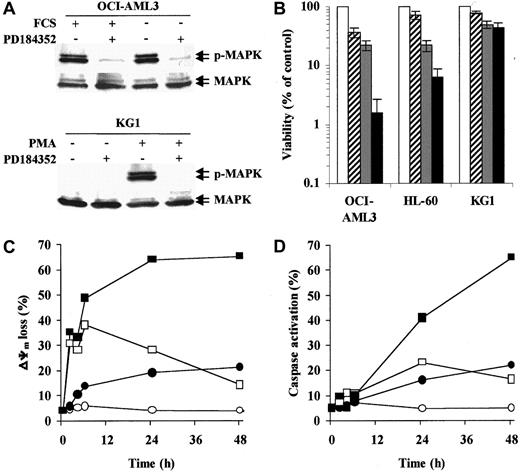
Three AML cell lines with different degrees of MAPK activation were used in this study. In OCI-AML3 (Figure1A) and HL-60 cells, which show high constitutive MAPK activity, PD184352 (10 μM) rapidly (1 hour) abrogated MAPK phosphorylation by inhibiting the upstream kinase MEK. Conversely, KG1 cells showed little, if any, constitutive MAPK phosphorylation. However, PD184352 was able to abrogate phorbol myristate acetate–induced MAPK activation in these cells (Figure 1A). In OCI-AML3 and HL-60 cell lines, the simultaneous disruption of Bax/Bcl-2 heterodimerization by HA14-1 and interruption of MEK-to-MAPK signaling by PD184352 resulted in a striking decrease in cell viability (48 hours; Figure 1B). Conversely, PD184352 did not significantly modify the response of KG1 cells to HA14-1 (Figure 1B).
MEK inhibition potentiates HA14-1 cytotoxicity and apoptosis in AML cell lines with constitutive MAPK activation.
(A) OCI-AML3 cells were cultured under standard conditions or in serum-free medium for 48 hours prior to exposure to PD184352 (10 μM) or vehicle control for 1 hour at 37°C. KG1 cells were pretreated with PD184352 (10 μM) or vehicle control for 1 hour at 37°C prior to exposure to phorbol myristate acetate (60 ng/mL) for 1 hour. Cells were then subjected to Western blot analysis for phosphorylated or total MAPK. Results from one experiment representative of 3 performed are shown. (B) The indicated AML cell lines were seeded at a starting concentration of 2 × 105 cells per milliliter and cultured in the presence of vehicle control (■), 1.25 μM PD184352 ( ), 12.5 μM HA14-1 (
), 12.5 μM HA14-1 ( ), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. After 48 hours, viable cells were counted by trypan blue exclusion. Results are expressed as a percentage of the viable cells in the vehicle control-treated group and represent the average ± SD of 4 independent experiments. (C) OCI-AML3 cells were seeded at a starting concentration of 2 × 105cells per milliliter and cultured in the presence of vehicle control (○), 1.25 μM PD184352 (●), 12.5 μM HA14-1 (■), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. At the indicated times, ΔΨm was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as percentage of cells with low ΔΨm. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments. (D) OCI-AML3 cells were cultured as described above. At the indicated times, caspase activation was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as the percentage of active caspase-positive cells. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments.
), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. After 48 hours, viable cells were counted by trypan blue exclusion. Results are expressed as a percentage of the viable cells in the vehicle control-treated group and represent the average ± SD of 4 independent experiments. (C) OCI-AML3 cells were seeded at a starting concentration of 2 × 105cells per milliliter and cultured in the presence of vehicle control (○), 1.25 μM PD184352 (●), 12.5 μM HA14-1 (■), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. At the indicated times, ΔΨm was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as percentage of cells with low ΔΨm. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments. (D) OCI-AML3 cells were cultured as described above. At the indicated times, caspase activation was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as the percentage of active caspase-positive cells. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments.
MEK inhibition potentiates HA14-1 cytotoxicity and apoptosis in AML cell lines with constitutive MAPK activation.
(A) OCI-AML3 cells were cultured under standard conditions or in serum-free medium for 48 hours prior to exposure to PD184352 (10 μM) or vehicle control for 1 hour at 37°C. KG1 cells were pretreated with PD184352 (10 μM) or vehicle control for 1 hour at 37°C prior to exposure to phorbol myristate acetate (60 ng/mL) for 1 hour. Cells were then subjected to Western blot analysis for phosphorylated or total MAPK. Results from one experiment representative of 3 performed are shown. (B) The indicated AML cell lines were seeded at a starting concentration of 2 × 105 cells per milliliter and cultured in the presence of vehicle control (■), 1.25 μM PD184352 ( ), 12.5 μM HA14-1 (
), 12.5 μM HA14-1 ( ), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. After 48 hours, viable cells were counted by trypan blue exclusion. Results are expressed as a percentage of the viable cells in the vehicle control-treated group and represent the average ± SD of 4 independent experiments. (C) OCI-AML3 cells were seeded at a starting concentration of 2 × 105cells per milliliter and cultured in the presence of vehicle control (○), 1.25 μM PD184352 (●), 12.5 μM HA14-1 (■), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. At the indicated times, ΔΨm was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as percentage of cells with low ΔΨm. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments. (D) OCI-AML3 cells were cultured as described above. At the indicated times, caspase activation was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as the percentage of active caspase-positive cells. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments.
), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. After 48 hours, viable cells were counted by trypan blue exclusion. Results are expressed as a percentage of the viable cells in the vehicle control-treated group and represent the average ± SD of 4 independent experiments. (C) OCI-AML3 cells were seeded at a starting concentration of 2 × 105cells per milliliter and cultured in the presence of vehicle control (○), 1.25 μM PD184352 (●), 12.5 μM HA14-1 (■), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. At the indicated times, ΔΨm was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as percentage of cells with low ΔΨm. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments. (D) OCI-AML3 cells were cultured as described above. At the indicated times, caspase activation was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as the percentage of active caspase-positive cells. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments.
We then analyzed the apoptotic response of OCI-AML3 cells to HA14-1 alone or in combination with PD184352. Consistent with the disruption of Bcl-2 function,20 HA14-1 (12.5 μM) induced rapid (2 hours) but transient mitochondrial depolarization, whereas caspase activation was detected only after 24 hours and in a smaller fraction of the cells (Figure 1C-D). Simultaneous treatment with PD184352 (1.25 μM) did not affect the early (2-6 hours) phase of HA14-1–induced mitochondrial depolarization but strikingly potentiated both loss of ΔΨm and caspase activation at later times (24-48 hours; Figure 1C-D). The apoptotic nature of the cell death triggered by simultaneous disruption of the Bcl-2 and MAPK pathways was confirmed by the exposure of phosphatidylserine on the outer leaflet of the plasma membrane and a decrease in the DNA content to sub-G1 levels (data not shown).
The kinetics of apoptotic events observed in response to HA14-1 alone is consistent with previous results showing that mitochondrial depolarization alone, in the absence of caspase activation, cannot trigger a full apoptotic response.21 Preliminary data from our group suggest that, in fact, AML cells that only lose ΔΨm in response to HA14-1 retain substantial in vitro clonogenic ability (M.M., unpublished results, December 2001). The late potentiation observed in response to simultaneous MEK inhibition is compatible with recent evidence that the MAPK pathway protects against apoptosis at the level of cytosolic caspase activation, strongly implicating an inhibitor of apoptosis proteinlike molecule.22 In this regard, we have recently demonstrated that MAPK inhibition specifically inhibits the expression of survivin in AML cells with constitutive MAPK activation within a compatible time frame.7,14 Alternatively, these 2 pathways may interact via MAPK-mediated phosphorylation of Bcl-2 itself and/or other Bcl-2 family members, such as BAD.9 23-25
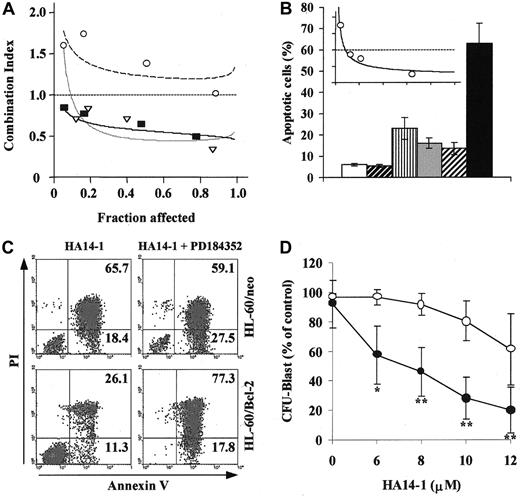
We further analyzed the pharmacologic interactions between HA14-1 and PD184352 using a fixed-ratio experimental design and found that the simultaneous disruption of both pathways resulted in the synergistic (CI < 1.0) induction of apoptosis in cell lines showing constitutive MAPK activation (OCI-AML3 and HL-60; Figure2A). Moreover, the exploration of a wider range of HA14-1 and PD184352 doses using different drug ratios (2:1 to 20:1) further confirmed the synergistic nature of this interaction (Table 1). Conversely, the combination of HA14-1 and PD184352 at a 10:1 ratio had a slightly antagonistic effect in KG1 cells (CI > 1.2; Figure 2A and Table 1).
MEK blockade and Bcl-2 inhibition have synergistic effects in AML cell lines and primary AML samples and overcome Bcl-2 overexpression-mediated resistance.
(A) OCI-AML3 (▿, dotted line), HL-60 (▪, solid line), and KG1 (○, dashed line) cells were cultured in the presence of escalating doses of HA14-1 (2.5-20 μM), PD184352 (0.016-10 μM), or combinations of the 2 agents at a 10:1 ratio (2.5/0.25, 5/0.5, 10/1, 15/1.5). After 48 hours, apoptosis was measured by sub-G1 DNA content. CI plots were then generated using the Calcusyn software (symbols represent actual data points for the combination). CI values of less than 1.0 indicate synergism. (B) HL-60 was cultured in the presence of vehicle control (■), 16 μM Bcl-2 NS (▨), 16 μM Bcl-2 AS (▥), 2 μM PD184352 ( ), Bcl-2 NS+PD184352 (
), Bcl-2 NS+PD184352 ( ), and Bcl-2 AS+PD184352 (▪). After 96 hours, cells were analyzed for DNA content. Results are presented as the percentage of cells with a sub-G1 DNA content and represent the average ± SD of 3 independent experiments. The inset shows the CI plot for the combination of escalating doses of Bcl-2 AS and PD184352 at a 8:1 ratio, obtained as described in panel A; the dotted line indicates CI = 1. (C) HL-60 cells stably transfected with either empty vector control (HL-60/neo) or Bcl-2 expression vector (HL-60/Bcl-2) were cultured in the presence of HA14-1 (20 μM) alone or in combination with PD184352 (1.2 μM). After 48 hours, cells were harvested and stained for annexin V binding (x-axis) while simultaneously assessing membrane integrity by propidium iodide staining (y-axis). Numbers indicate the percentage of cells in the corresponding quadrant. Results from one experiment representative of 2 performed are shown. (D) Bone marrow cells obtained at diagnosis (all but 1 patient progressing from myelodysplastic syndrome (MDS)) from 5 AML (M0, complex karyotype; M2, diploid; M4, inv(16); M4, diploid; previous MDS, monosomy 7) patients with more than 50% (median 61%, range 51%-79%) blasts were subjected to AML blast colony assay15 in the presence of the indicated doses of HA14-1, alone (○) or in combination with a fixed dose of PD184352 (1.25 μM, ●). Results shown represent the average ± SD of the results obtained in each individual patient and are expressed as a percentage of the colony-forming unit blast in the untreated control. The mean ± SD numbers of colonies in the untreated control for each individual patient were 608 ± 29, 627 ± 22, 584 ± 27, 392 ± 32, and 643 ± 18, respectively (*P = .015 and **P < .008 by Studentt test).
), and Bcl-2 AS+PD184352 (▪). After 96 hours, cells were analyzed for DNA content. Results are presented as the percentage of cells with a sub-G1 DNA content and represent the average ± SD of 3 independent experiments. The inset shows the CI plot for the combination of escalating doses of Bcl-2 AS and PD184352 at a 8:1 ratio, obtained as described in panel A; the dotted line indicates CI = 1. (C) HL-60 cells stably transfected with either empty vector control (HL-60/neo) or Bcl-2 expression vector (HL-60/Bcl-2) were cultured in the presence of HA14-1 (20 μM) alone or in combination with PD184352 (1.2 μM). After 48 hours, cells were harvested and stained for annexin V binding (x-axis) while simultaneously assessing membrane integrity by propidium iodide staining (y-axis). Numbers indicate the percentage of cells in the corresponding quadrant. Results from one experiment representative of 2 performed are shown. (D) Bone marrow cells obtained at diagnosis (all but 1 patient progressing from myelodysplastic syndrome (MDS)) from 5 AML (M0, complex karyotype; M2, diploid; M4, inv(16); M4, diploid; previous MDS, monosomy 7) patients with more than 50% (median 61%, range 51%-79%) blasts were subjected to AML blast colony assay15 in the presence of the indicated doses of HA14-1, alone (○) or in combination with a fixed dose of PD184352 (1.25 μM, ●). Results shown represent the average ± SD of the results obtained in each individual patient and are expressed as a percentage of the colony-forming unit blast in the untreated control. The mean ± SD numbers of colonies in the untreated control for each individual patient were 608 ± 29, 627 ± 22, 584 ± 27, 392 ± 32, and 643 ± 18, respectively (*P = .015 and **P < .008 by Studentt test).
MEK blockade and Bcl-2 inhibition have synergistic effects in AML cell lines and primary AML samples and overcome Bcl-2 overexpression-mediated resistance.
(A) OCI-AML3 (▿, dotted line), HL-60 (▪, solid line), and KG1 (○, dashed line) cells were cultured in the presence of escalating doses of HA14-1 (2.5-20 μM), PD184352 (0.016-10 μM), or combinations of the 2 agents at a 10:1 ratio (2.5/0.25, 5/0.5, 10/1, 15/1.5). After 48 hours, apoptosis was measured by sub-G1 DNA content. CI plots were then generated using the Calcusyn software (symbols represent actual data points for the combination). CI values of less than 1.0 indicate synergism. (B) HL-60 was cultured in the presence of vehicle control (■), 16 μM Bcl-2 NS (▨), 16 μM Bcl-2 AS (▥), 2 μM PD184352 ( ), Bcl-2 NS+PD184352 (
), Bcl-2 NS+PD184352 ( ), and Bcl-2 AS+PD184352 (▪). After 96 hours, cells were analyzed for DNA content. Results are presented as the percentage of cells with a sub-G1 DNA content and represent the average ± SD of 3 independent experiments. The inset shows the CI plot for the combination of escalating doses of Bcl-2 AS and PD184352 at a 8:1 ratio, obtained as described in panel A; the dotted line indicates CI = 1. (C) HL-60 cells stably transfected with either empty vector control (HL-60/neo) or Bcl-2 expression vector (HL-60/Bcl-2) were cultured in the presence of HA14-1 (20 μM) alone or in combination with PD184352 (1.2 μM). After 48 hours, cells were harvested and stained for annexin V binding (x-axis) while simultaneously assessing membrane integrity by propidium iodide staining (y-axis). Numbers indicate the percentage of cells in the corresponding quadrant. Results from one experiment representative of 2 performed are shown. (D) Bone marrow cells obtained at diagnosis (all but 1 patient progressing from myelodysplastic syndrome (MDS)) from 5 AML (M0, complex karyotype; M2, diploid; M4, inv(16); M4, diploid; previous MDS, monosomy 7) patients with more than 50% (median 61%, range 51%-79%) blasts were subjected to AML blast colony assay15 in the presence of the indicated doses of HA14-1, alone (○) or in combination with a fixed dose of PD184352 (1.25 μM, ●). Results shown represent the average ± SD of the results obtained in each individual patient and are expressed as a percentage of the colony-forming unit blast in the untreated control. The mean ± SD numbers of colonies in the untreated control for each individual patient were 608 ± 29, 627 ± 22, 584 ± 27, 392 ± 32, and 643 ± 18, respectively (*P = .015 and **P < .008 by Studentt test).
), and Bcl-2 AS+PD184352 (▪). After 96 hours, cells were analyzed for DNA content. Results are presented as the percentage of cells with a sub-G1 DNA content and represent the average ± SD of 3 independent experiments. The inset shows the CI plot for the combination of escalating doses of Bcl-2 AS and PD184352 at a 8:1 ratio, obtained as described in panel A; the dotted line indicates CI = 1. (C) HL-60 cells stably transfected with either empty vector control (HL-60/neo) or Bcl-2 expression vector (HL-60/Bcl-2) were cultured in the presence of HA14-1 (20 μM) alone or in combination with PD184352 (1.2 μM). After 48 hours, cells were harvested and stained for annexin V binding (x-axis) while simultaneously assessing membrane integrity by propidium iodide staining (y-axis). Numbers indicate the percentage of cells in the corresponding quadrant. Results from one experiment representative of 2 performed are shown. (D) Bone marrow cells obtained at diagnosis (all but 1 patient progressing from myelodysplastic syndrome (MDS)) from 5 AML (M0, complex karyotype; M2, diploid; M4, inv(16); M4, diploid; previous MDS, monosomy 7) patients with more than 50% (median 61%, range 51%-79%) blasts were subjected to AML blast colony assay15 in the presence of the indicated doses of HA14-1, alone (○) or in combination with a fixed dose of PD184352 (1.25 μM, ●). Results shown represent the average ± SD of the results obtained in each individual patient and are expressed as a percentage of the colony-forming unit blast in the untreated control. The mean ± SD numbers of colonies in the untreated control for each individual patient were 608 ± 29, 627 ± 22, 584 ± 27, 392 ± 32, and 643 ± 18, respectively (*P = .015 and **P < .008 by Studentt test).
The specificity of the interaction between Bcl-2 and the MEK/MAPK module was further studied using liposome-delivered Bcl-2 AS.5 As shown in Figure 2B, the combination of Bcl-2 AS and PD184352 at a 8:1 ratio caused substantially more apoptosis than either agent alone. Isobologram analysis confirmed that the proapoptotic interaction between Bcl-2 AS and PD184352 was indeed synergistic (CI = 0.36 ± 0.03; Figure 2B inset). Moreover, while Bcl-2 overexpression produced by stable gene transfer increased the resistance of HL-60– to HA14-1–induced apoptosis (Figure 2C, left panels), this effect was overcome by simultaneous treatment with PD184352 (Figure 2C, right panels), which shifted the 90% effective dose (ED90) of HA14-1 from 32 μM to 22 μM (ED90 in parental HL-60 cells, 23 μM).
Although widely used and undoubtedly useful as a model to conduct mechanistic studies, culture-adapted cell lines may not accurately reflect the behavior of patient-derived cancer cells.26 We therefore sought to confirm our cell line findings in primary AML samples. In all bone marrow samples tested in the AML blast clonogenic assay (n = 5), simultaneous exposure to a fixed concentration of PD184352 (1.25 μM) significantly potentiated the colony inhibitory effect of escalating doses of HA14-1 (6-12 μM; Figure 2D).
With rare exceptions, neoplastic cell growth is the result of multiple genetic alterations27; therefore, any new clinically successful therapeutic strategies will most likely draw on the mechanism-based manipulation of multiple, crosstalking pathways involved in growth and survival control. From the standpoint of AML, our findings indicate that the simultaneous disruption of both the Bcl-2 and MAPK pathways synergistically induces apoptosis in cells showing constitutive MAPK activation. Because we have recently demonstrated that patients with AML with antiapoptotic Bax/Bcl-2 ratios and activated MAPK have a uniformly poor prognosis (S.M.K., manuscript submitted, September 2001), the disruption of the antiapoptotic crosstalk between the Bcl-2 and MAPK pathways could be usefully exploited for therapeutic purposes in a population of patients with AML that is currently the least responsive to conventional treatment strategies.
The authors thank Judith S. Sebolt-Leopold (Pfizer Global Research and Development) for kindly providing PD184352, Jennifer Jones and Matthew Womble for their help with primary AML samples, and Rosemarie Lauzon for her assistance with the manuscript.
Supported in part by NIH grants (P01 CA55164, P01 CA49639, CA16672), the Keck Foundation, and the Stringer Professorship for Cancer Treatment and Research (to M.A.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Andreeff, Section of Molecular Hematology and Therapy, Department of Blood and Marrow Transplantation, The University of Texas M. D. Anderson Cancer Center, 1400 Holcombe Blvd, Box 448, Houston, TX 77030; e-mail: mandreef@mdanderson.org.


 ), 12.5 μM HA14-1 (
), 12.5 μM HA14-1 ( ), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. After 48 hours, viable cells were counted by trypan blue exclusion. Results are expressed as a percentage of the viable cells in the vehicle control-treated group and represent the average ± SD of 4 independent experiments. (C) OCI-AML3 cells were seeded at a starting concentration of 2 × 105cells per milliliter and cultured in the presence of vehicle control (○), 1.25 μM PD184352 (●), 12.5 μM HA14-1 (■), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. At the indicated times, ΔΨm was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as percentage of cells with low ΔΨm. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments. (D) OCI-AML3 cells were cultured as described above. At the indicated times, caspase activation was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as the percentage of active caspase-positive cells. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments.
), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. After 48 hours, viable cells were counted by trypan blue exclusion. Results are expressed as a percentage of the viable cells in the vehicle control-treated group and represent the average ± SD of 4 independent experiments. (C) OCI-AML3 cells were seeded at a starting concentration of 2 × 105cells per milliliter and cultured in the presence of vehicle control (○), 1.25 μM PD184352 (●), 12.5 μM HA14-1 (■), or a combination of PD184352 and HA14-1 (▪). Vehicle control and PD184352-treated cells were also exposed to 12.5 μM of an inactive compound structurally related to HA14-1. At the indicated times, ΔΨm was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as percentage of cells with low ΔΨm. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments. (D) OCI-AML3 cells were cultured as described above. At the indicated times, caspase activation was assessed by flow cytometry. Results represent the average of triplicate measurements (10 000 total events for each measurement) and are expressed as the percentage of active caspase-positive cells. SE was constantly less than 2% and is therefore omitted for clarity. Comparable results were obtained in 2 other independent experiments.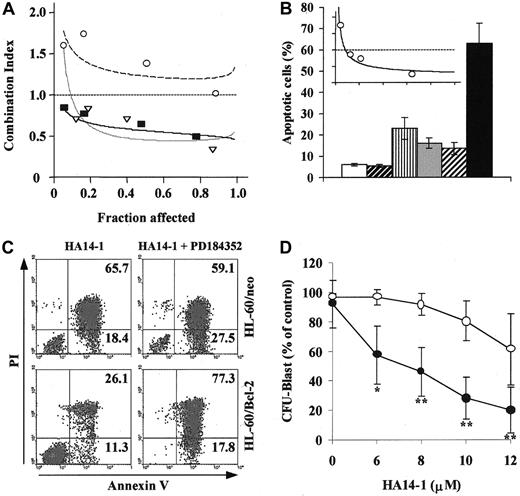
 ), Bcl-2 NS+PD184352 (
), Bcl-2 NS+PD184352 (