Abstract
Long-term clinical remissions of leukemia, after allogeneic hematopoietic stem cell transplantation, depend on alloreactive memory T cells able to self-renew and differentiate into antileukemia effectors. This is counterbalanced by detrimental graft-versus-host disease (GVHD). Induction of a selective suicide in donor T cells is a current gene therapy approach to abrogate GVHD. Unfortunately, genetic modification reduces alloreactivity of lymphocytes. This associates with an effector memory (TEM) phenotype of gene-modified lymphocytes and may limit antileukemia effect. We hypothesized that alloreactivity of gene-modified lymphocytes segregates with the central memory (TCM) phenotype. To this, we generated suicide gene–modified TCM lymphocytes with a retroviral vector after CD28 costimulation and culture with IL-2, IL-7, or a combination of IL-7 and IL-15. In vitro, suicide gene–modified TCM cells self-renewed upon alloantigen stimulation and resisted activation-induced cell death. In a humanized mouse model, only suicide gene–modified T cells cultured with IL-7 and IL-15 persisted, differentiated in TEM cells, and were as potent as unmanipulated lymphocytes in causing GVHD. GVHD was halted through the activation of the suicide gene machinery. These results warrant the use of suicide gene–modified TCM cells cultured with IL-7 and IL-15 for the safe exploitation of the alloreactive response against cancer.
Introduction
The transfer of antitumor immunotherapeutics into patients is a promising approach for the treatment of cancer.1 These include monoclonal antibodies specific for molecules expressed on the surface of tumor cells and tumor-reactive T lymphocytes. Monoclonal antibodies recognize and destroy tumor cells or block their proliferation in vivo and have been proven to prolong survival of cancer patients. Tumor-reactive T lymphocytes selectively migrate and accumulate at the site of disease, where they exert their effector function. The transfer of high numbers of tumor-reactive effector T lymphocytes (adoptive T-cell therapy) may overcome cancer-related immunosuppression and mediate tumor regression.2,3 The greatest advantage of adoptive T-cell therapy over conventional treatments, including radiochemotherapy, specific inhibitors of signal transduction, or monoclonal antibodies, lies in the promise of long-term protection against cancer.
Long-term cancer immune surveillance associates with long-living tumor-reactive memory T lymphocytes. The generation of memory T lymphocytes has been extensively studied in mouse models of viral infection.4 During an acute infection, clonal expansion of virus-specific T lymphocytes and differentiation into effectors either clear the virus or confine it, thus limiting tissue damage.5 As the acute infection resolves, memory T lymphocytes appear. Virus-specific memory T lymphocytes can survive in conditions of limited or no access to the antigen. They are devoid of effector functions but can readily differentiate into effectors upon antigen re-exposure.6 Antitumor and antiviral immune responses share many features. The degree of differentiation of adoptively transferred tumor-reactive CD8+ T lymphocytes has been shown to inversely correlate with their efficacy against melanoma in vivo.7 Upon antigen encounter, primate, including human, T lymphocytes undergo a developmental program from naive (TNA) to central memory (TCM) and effector memory (TEM) cells. TNA cells are homogenously CD45RA+CD62L+, whereas memory lymphocytes are either CD45RA−CD62L+ (TCM) or CD45RA−CD62L− (TEM).8 As T lymphocytes differentiate into TEM cells, they lose proliferative and homing potential to lymph nodes and become sensitive to activation-induced cell death (AICD). Conversely, TCM cells maintain homing potential to lymph nodes and the ability to survive and proliferate in the absence of antigen.8,9 Recently, Berger et al showed that the adoptive transfer of antigen-specific CD8+ T-cell clones from TCM but not from TEM lymphocytes can provide persistent T-cell memory in vivo.10
In allogeneic hematopoietic stem cell transplantation (allo-HCT), alloreactive donor T lymphocytes sustain a therapeutic graft-versus-malignancy effect.11,12 Unfortunately, allo-HCT is limited by graft-versus-host disease (GVHD), which is often lethal. In mouse models, alloreactivity segregates with TNA cells.13 Nevertheless, the progression of the alloreactive response depends on memory T lymphocytes, able to self-renew and to differentiate into effectors.14 Others and we showed that genetic modification with a retroviral vector (RV) encoding for a suicide gene allows for an operational separation of the graft-versus-leukemia (GVL) effect from GVHD.15-18 We treated patients suffering from hematologic malignancies with donor T lymphocytes modified to express the thymidine kinase (TK) suicide gene. TK+ lymphocytes become selectively sensitive to ganciclovir (GCV)–mediated cell death, enabling their in vivo elimination. GCV administration blocked the progression of GVHD, while sparing a significant GVL effect.15,16 Several reports suggest that genetic modification with RV reduces the T-cell life span, proliferative potential, and alloreactivity.19,20 This is possibly because current clinical protocols for the generation of gene-modified lymphocytes are biased toward TEM cells.18 RV-mediated genetic modification after CD28 costimulation and low-dose IL-2 generates TK+ TCM cells that are more potent than the TEM counterpart in a xenograft in vivo model of GVHD.21 Nevertheless, TK+ TCM cells cultured with IL-2 are less reactive than unmanipulated lymphocytes.
Cytokine requirements for memory T-cell alloreactivity are poorly understood. In vivo, the maintenance of the pool of TCM lymphocytes depends on the disposal of homeostatic γ-chain cytokines promoting memory T-cell survival and proliferation, even in the absence of antigen.22 Among these, IL-7 is involved mainly in memory T-cell survival,23-25 whereas IL-15 induces lymphocyte proliferation.26,27 In a mouse model of CD8+ T cell–mediated GVHD, IL-15 was critically required to generate candidate memory stem T cells able to self-renew and differentiate into alloreactive effectors.14 Herein, we report that IL-7 and IL-15 are required for the expansion of human alloreactive TK+ TCM cells able to self-renew, resist activation-induced cell death in vitro, and cause GVHD in a fully humanized mouse model. Of interest, TK+ TCM cells cultured with IL-7 and IL-15 proved as potent as unmanipulated lymphocytes in mediating alloreactivity. Moreover, we demonstrate that activation of the suicide gene machinery can block the progression of GVHD induced by TK+ TCM cells.
Methods
Genetic modification and culture of human T lymphocytes
Approval for these studies was obtained from the San Raffaele Scientific Institutional Review Board. Healthy donors' peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation on a density gradient (Lymphoprep; Fresenius, Oslo, Norway), cultured in RPMI1640 medium (GIBCO-BRL, Gaithersburg, MD) supplemented with antibiotics, glutamine, and 10% FBS (BioWhittaker-Italia, Milano, Italy), and activated by 30 ng/mL anti-CD3 antibodies (OKT3; OrthoBiotech, Raritan, NJ) or anti-CD3/CD28–conjugated magnetic beads (ClinExVivo CD3/CD28; Invitrogen, Carlsbad, CA) in 3:1 bead/T-cell ratio at the initial day of culture. T lymphocytes activated by OKT3 were cultured with 600 IU/mL rhIL-2 (Chiron, Emeryville, CA), according to the protocol we are currently using in the clinic18 (F.C., C. Bonini, A.B., C. Bordignon, manuscript submitted, August 2008), whereas T lymphocytes activated by baCD3/CD28 were cultured: (1) in the absence of cytokines; (2) with rhIL-2 at 200 IU/mL; (3) with rhIL-7 at 5 ng/mL (Peprotech, London, United Kingdom); and (4) with both rhIL-7 and rhIL-15 at 5 ng/mL each (Peprotech). Cytokines were added every 3 days. At days 2 and 3 of culture, cells were transduced by the SFCMM-3 Mut2 retroviral vector (MolMed, Milan, Italy).21,28,29 The SFCMM-3 encodes TK gene and a truncated low-affinity nerve growth factor receptor (ΔLNGFR) gene. SFCMM-3 Mut2 is a splice-corrected version of SFCMM-3, in which a single base silent mutation has been introduced in the TK gene.28 Viral supernatant was produced in large scale up to 75 L/batch. The production process was optimized in terms of producer cell culture conditions and kinetics of supernatant harvesting. ΔLNGFR+ T lymphocytes were positively selected at day 7 by mouse anti-LNGFR antibody (MolMed) and antimouse antibody-conjugated magnetic beads (Dynabeads Sheep-anti Mouse IgG; Invitrogen) according to the manufacturer's protocol. Then, ΔLNGFR+ T lymphocytes were expanded for 3 days. Transduction efficiency was expressed as the percentage of ΔLNGFR+ cells at day 7, and functional phenotypes of purified TK+ lymphocytes were examined at day 10. At day 10, transduced cells were tested for alloreactivity in vitro and in vivo as described in “Results.”
Flow cytometry
For T-lymphocyte phenotyping we used FITC, PE, PerCP, or APC-conjugated antibodies directed to human CD3, CD4, CD8, CD45RA, CD62L, CCR7, CD27, CD28, CD25, CD122, CD127, LNGFR, IL-2, IFNγ, and mouse CD45 (BD Biosciences, San Jose, CA). Cells (5 × 105) were incubated with antibodies for 25 minutes at 4°C and washed with PBS. To-Pro-3 (Invitrogen) was added to some samples at 5 μM to detect dead cells. Samples were eventually run through a FACS Calibur or FACS Canto II flow cytometer (BD, Mountain View, CA). For intracellular cytokine staining, 106 cells were stimulated with 50 ng/mL PMA (Sigma-Aldrich, St Louis, MO) and 1 μg/mL ionomycin (Sigma-Aldrich). After 4 hours, 10 μg/mL brefeldin A (Sigma-Aldrich) was added. Two hours later, cells were stained with surface antibodies and fixed with 1% paraformaldehyde. Intracellular staining was then performed with anti–IL-2 and IFNγ antibodies in PBS containing 2% FBS and 0.05% saponin (Sigma-Aldrich).
Alloreactivity and activation induced cell death sensitivity of human TK+ lymphocytes
Alloreactivity of TK+ lymphocyte was analyzed at day 10 after TCR stimulation. ΔLNGFR+ and unmanipulated autologous T lymphocytes were isolated (magnetic-activated cell sorting [MACS] pan CD3 isolation kit II; Miltenyi Biotec, Bologna, Italy) according to the manufacturer's protocol, then stained by carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) at 0.5 μM. CFSE-labeled cells (106) were stimulated with 3000 cGy irradiated allogeneic peripheral blood mononuclear cells (PBMCs) at 1:1 ratio. At day 7, viable cells were harvested and restimulated the same way. After 6 days from each stimulation, cells were counted and analyzed for CFSE dilution by flow cytometry.
Activation-induced cell death (AICD) assay was performed by culturing CFSE-labeled T lymphocytes on plastic-bound OKT3 at increasing concentrations (0.01, 0.1, and 1 μg/mL). At day 3, 20 IU/mL IL-2 was added and cell death was analyzed by flow cytometry at day 6 after exposure to To-Pro-3.
Human skin grafting and T-lymphocyte infusion in NOD/Scid mice
The experimental protocol was approved by the Institutional Committee for animal studies at all participating institutions. Full-thickness human skin was obtained from patients enrolled in abdominal surgery, after informed consent was obtained in accordance with the Declaration of Helsinki. Six- to 8-week-old female nonobese diabetic/severe combined immunodeficient (NOD/Scid) mice (Charles-River Italia, Calco, Italy) were anesthetized with 3,3,3 tribromoethanol (Avertin; Sigma-Aldrich) and then received a transplant of 50 mm2 human skin subcutaneously. After 10 days, mice received 1 mg anti–mouse IL-2/15Rβ mAb (TMβ-1, kindly provided by Prof Tanaka) intraperitoneally to neutralize natural killer (NK)–cell activity. The following day (day 11), mice were irradiated with 300 cGy and then injected via the tail vein with human transduced T lymphocytes (harvested at day 10 of culture) or with purified untransduced T cells from a fully mismatched donor.
Human T-lymphocyte engraftment and allogeneic skin GVHD
At week 1 and 2 after T-cell injection, human T-cell chimerism in murine blood was expressed as: (% of human CD3 lymphocytes/[% of murine CD45 cells + human CD3 lymphocytes]). Mice with more than 2% human chimerism were included for further blood and human skin analyses. Mice were killed at weeks 2 to 3 after the infusion and human skin was removed, fixed by formalin, embedded in paraffin, cut in sections, and stained with H&E for histologic evaluation. Immunohistochemical staining was performed with monoclonal antibodies to human CD3 (Dako, Glostrup, Denmark). Antibody binding was revealed with the avidin/biotin peroxidase complex method and sections were counterstained. Skin GvHD was blindly graded by 2 pathologists according to pathologic criteria. All images were acquired with a Zeiss Axioskop Plus microscope (Zeiss, Heidelberg, Germany) with Plan Neofluar 40×/0.75 and 10×/0.75 NA objective lenses and no imaging medium. Pictures were taken with a Zeiss Axiocam HRC.
GCV administration and evaluation of response
Three to 6 days after T-cell injection, mice were treated with GCV or PBS. Briefly, Alzet pumps (Durect, Cupertino, CA) were filled with 200 μL GCV (25 mg/mL) or PBS and implanted subcutaneously to ensure drug release at a constant rate. At day 7 of treatment, circulating human T lymphocytes were quantified and analyzed by flow cytometry and human skin grafts were removed and histologically analyzed as previously described.
Statistical analysis
All statistics in this study were performed by Excel 2003 (Microsoft, Redmond, WA) and Statcel2 (OMS publish, Saitama, Japan). Tukey or Scheffe F test following ANOVA, and Mann-Whitney U test following Kruskal-Wallis test or Scheffe F test following Freidman test were performed for parametric and nonparametric data, respectively. Pathologic GVHD grades were analyzed by χ2 or Fisher exact probability test.
Results
Common γ-chain cytokines expand gene-modified human TCM lymphocytes after CD28 costimulation
We initially compared the ability of common γ-chain cytokines to expand suicide gene–modified TCM lymphocytes after CD28 costimulation. T lymphocytes from healthy volunteers were stimulated with cell-sized beads conjugated with antibodies to CD3 and CD28 (baCD3/CD28),21,30 exposed to the SFCMM-3 Mut2 retroviral vector encoding for the TK suicide gene and the ΔLNGFR gene. Cells were cultured in the presence of low-dose IL-2 (200 IU/mL), IL-7 (5 ng/mL), or a combination of IL-7 (5 ng/mL) and IL-15 (5 ng/mL) or in the absence of cytokines. We did not test IL-15 alone, since we previously found that this drives the generation of TEM cells.31 As control, we used the current clinical protocol for the generation of TK+ lymphocytes, which is based on T-cell stimulation with anti-CD3 antibody (aCD3) and culture with high-dose IL-2 (600 IU/mL).15,16 As shown in Figure 1A, CD28 costimulation enabled higher transduction efficiency than CD3 stimulation alone, regardless of the cytokine used. The average yield of TK+ cells at day 10 of culture was 1.3 (± 0.8) for aCD3+ IL-2, 2.5 (± 1.6) for baCD3/CD28+ IL-2, 2.3 (± 1.6) for baCD3/CD28+ IL-7, 2.7 (± 2.0) for baCD3/CD28+ IL-7/IL-15, and 1.7 (± 1.5) for baCD3/CD28 alone (all baCD3/CD28+ cytokines conditions vs aCD3+ IL-2, P < .05). Within the following 2 weeks of culture, TK+ lymphocytes generated with CD28 costimulation and low-dose IL-7 and IL-15 expanded significantly more than TK+ lymphocytes generated with any other condition. Common γ-chain cytokines were required for T-cell expansion after CD28 costimulation, since in their absence cells did not survive beyond week 2 (Figure 1B). Based on this observation, T-cell culture in the absence of γ-chain cytokines was not further explored. CD28 costimulation targeted the transgene to both CD4+ and CD8+ lymphocytes, thus maintaining the original CD4/CD8 ratio (Figure 1C). Conversely, CD3 stimulation alone preferentially expanded CD8+TK+ lymphocytes. The majority of TK+ lymphocytes generated with CD28 costimulation had a TCM phenotype (CD45RA−CD62L+, Figure 1D). On the contrary, TK+ lymphocytes generated with CD3 stimulation alone were mainly TEM cells (CD45RA−CD62L−). The effect was independent from the cytokine used (all conditions with baCD3/CD28 vs aCD3+ IL-2, P < .01). To further confirm the stage of differentiation of TK+ lymphocytes, we assessed the expression of CD27 and CD28.32 TK+ lymphocytes obtained after CD28 costimulation were homogenously CD27+ CD28+, whereas a significant fraction of TK+ lymphocytes obtained after CD3 stimulation alone had lost CD28 expression (Figure 1E). To evaluate the functional phenotype of TK+ lymphocytes, we studied their intracellular cytokine profile after polyclonal activation. The majority of TK+ lymphocytes generated with CD28 costimulation were early differentiated IL-2+ IFN-γ− cells, whereas TK+ lymphocytes after CD3 stimulation had a mixed phenotype (Figure 1F).
Retroviral transduction after baCD3/CD28 activation and culture with γ-chain cytokines results in central memory TK+ lymphocytes. (A) PBMCs were stimulated with either baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or baCD3/CD28 only (▦). At 48 and 72 hours after stimulation, cells were transduced with the cell-free supernatant carrying the retroviral vector SCFMM-3 Mut2. Transduction efficiency was measured by flow cytometry at day 7. Averages plus or minus SD of 9 independent experiments from different donors are shown. **P < .01 versus all other samples. (B) TK+ lymphocytes were cultured until day 21. Cytokines were added every 3 to 4 days. Immune-selected cells were counted with trypan blue exclusion at days 7, 9, 15, and 21. To evaluate and compare TK+ lymphocyte expansion, while avoiding biases related to variable transduction efficiency, cell expansion was calculated according to the following formula: (no. of cells at day X/no. of cells at day 7). baCD3/CD28+ IL-7/IL-15 (◆), baCD3/CD28+ IL-7 (
), aCD3+ IL-2 (□), or baCD3/CD28 only (▦). At 48 and 72 hours after stimulation, cells were transduced with the cell-free supernatant carrying the retroviral vector SCFMM-3 Mut2. Transduction efficiency was measured by flow cytometry at day 7. Averages plus or minus SD of 9 independent experiments from different donors are shown. **P < .01 versus all other samples. (B) TK+ lymphocytes were cultured until day 21. Cytokines were added every 3 to 4 days. Immune-selected cells were counted with trypan blue exclusion at days 7, 9, 15, and 21. To evaluate and compare TK+ lymphocyte expansion, while avoiding biases related to variable transduction efficiency, cell expansion was calculated according to the following formula: (no. of cells at day X/no. of cells at day 7). baCD3/CD28+ IL-7/IL-15 (◆), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or baCD3/CD28 only (black square with dotted line). Median of 3 independent experiments from different donors is shown. *P < .05; **P < .01 versus baCD3/CD28+ IL-7/IL-15. (C) CD4/CD8 ratio was measured on transduced lymphocytes by flow cytometry at day 10. Averages plus or minus SD of 9 independent experiments from 9 independent donors are shown. **P < .01 versus all other samples. (D) Expression of CD45RA and CD62L was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of TNA (CD45RA+CD62L+, ▥), TCM (CD45RA−CD62L+, ▨), TEM (CD45RA−CD62L−, ▩), and TE (CD45RA+CD62L− ▩) lymphocytes on CD3 TK+ lymphocytes are shown. N = 6 or more per group from 10 independent experiments with 10 independent donors. **P < .01, versus all other samples. (E) Expression of CD27 and CD28 was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of CD27+CD28+ (▨), CD27+CD28− (▩), CD27−CD28+ (▥), and CD27−CD28− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 or more per group from 9 independent experiments with 9 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2. (F) At day 10, TK+ lymphocytes were stimulated by PMA/ionomycin and analyzed by flow cytometry for IFNγ and IL-2 production. Averages plus or minus SD of IFNγ−IL-2+ (▨), IFNγ+IL-2+ (▩), and IFNγ+IL-2− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 per group from 5 independent experiments with 5 independent donors. *P < .05; **P < .01 versus aCD3+ IL-2.
), aCD3+ IL-2 (□), or baCD3/CD28 only (black square with dotted line). Median of 3 independent experiments from different donors is shown. *P < .05; **P < .01 versus baCD3/CD28+ IL-7/IL-15. (C) CD4/CD8 ratio was measured on transduced lymphocytes by flow cytometry at day 10. Averages plus or minus SD of 9 independent experiments from 9 independent donors are shown. **P < .01 versus all other samples. (D) Expression of CD45RA and CD62L was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of TNA (CD45RA+CD62L+, ▥), TCM (CD45RA−CD62L+, ▨), TEM (CD45RA−CD62L−, ▩), and TE (CD45RA+CD62L− ▩) lymphocytes on CD3 TK+ lymphocytes are shown. N = 6 or more per group from 10 independent experiments with 10 independent donors. **P < .01, versus all other samples. (E) Expression of CD27 and CD28 was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of CD27+CD28+ (▨), CD27+CD28− (▩), CD27−CD28+ (▥), and CD27−CD28− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 or more per group from 9 independent experiments with 9 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2. (F) At day 10, TK+ lymphocytes were stimulated by PMA/ionomycin and analyzed by flow cytometry for IFNγ and IL-2 production. Averages plus or minus SD of IFNγ−IL-2+ (▨), IFNγ+IL-2+ (▩), and IFNγ+IL-2− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 per group from 5 independent experiments with 5 independent donors. *P < .05; **P < .01 versus aCD3+ IL-2.
Retroviral transduction after baCD3/CD28 activation and culture with γ-chain cytokines results in central memory TK+ lymphocytes. (A) PBMCs were stimulated with either baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or baCD3/CD28 only (▦). At 48 and 72 hours after stimulation, cells were transduced with the cell-free supernatant carrying the retroviral vector SCFMM-3 Mut2. Transduction efficiency was measured by flow cytometry at day 7. Averages plus or minus SD of 9 independent experiments from different donors are shown. **P < .01 versus all other samples. (B) TK+ lymphocytes were cultured until day 21. Cytokines were added every 3 to 4 days. Immune-selected cells were counted with trypan blue exclusion at days 7, 9, 15, and 21. To evaluate and compare TK+ lymphocyte expansion, while avoiding biases related to variable transduction efficiency, cell expansion was calculated according to the following formula: (no. of cells at day X/no. of cells at day 7). baCD3/CD28+ IL-7/IL-15 (◆), baCD3/CD28+ IL-7 (
), aCD3+ IL-2 (□), or baCD3/CD28 only (▦). At 48 and 72 hours after stimulation, cells were transduced with the cell-free supernatant carrying the retroviral vector SCFMM-3 Mut2. Transduction efficiency was measured by flow cytometry at day 7. Averages plus or minus SD of 9 independent experiments from different donors are shown. **P < .01 versus all other samples. (B) TK+ lymphocytes were cultured until day 21. Cytokines were added every 3 to 4 days. Immune-selected cells were counted with trypan blue exclusion at days 7, 9, 15, and 21. To evaluate and compare TK+ lymphocyte expansion, while avoiding biases related to variable transduction efficiency, cell expansion was calculated according to the following formula: (no. of cells at day X/no. of cells at day 7). baCD3/CD28+ IL-7/IL-15 (◆), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or baCD3/CD28 only (black square with dotted line). Median of 3 independent experiments from different donors is shown. *P < .05; **P < .01 versus baCD3/CD28+ IL-7/IL-15. (C) CD4/CD8 ratio was measured on transduced lymphocytes by flow cytometry at day 10. Averages plus or minus SD of 9 independent experiments from 9 independent donors are shown. **P < .01 versus all other samples. (D) Expression of CD45RA and CD62L was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of TNA (CD45RA+CD62L+, ▥), TCM (CD45RA−CD62L+, ▨), TEM (CD45RA−CD62L−, ▩), and TE (CD45RA+CD62L− ▩) lymphocytes on CD3 TK+ lymphocytes are shown. N = 6 or more per group from 10 independent experiments with 10 independent donors. **P < .01, versus all other samples. (E) Expression of CD27 and CD28 was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of CD27+CD28+ (▨), CD27+CD28− (▩), CD27−CD28+ (▥), and CD27−CD28− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 or more per group from 9 independent experiments with 9 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2. (F) At day 10, TK+ lymphocytes were stimulated by PMA/ionomycin and analyzed by flow cytometry for IFNγ and IL-2 production. Averages plus or minus SD of IFNγ−IL-2+ (▨), IFNγ+IL-2+ (▩), and IFNγ+IL-2− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 per group from 5 independent experiments with 5 independent donors. *P < .05; **P < .01 versus aCD3+ IL-2.
), aCD3+ IL-2 (□), or baCD3/CD28 only (black square with dotted line). Median of 3 independent experiments from different donors is shown. *P < .05; **P < .01 versus baCD3/CD28+ IL-7/IL-15. (C) CD4/CD8 ratio was measured on transduced lymphocytes by flow cytometry at day 10. Averages plus or minus SD of 9 independent experiments from 9 independent donors are shown. **P < .01 versus all other samples. (D) Expression of CD45RA and CD62L was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of TNA (CD45RA+CD62L+, ▥), TCM (CD45RA−CD62L+, ▨), TEM (CD45RA−CD62L−, ▩), and TE (CD45RA+CD62L− ▩) lymphocytes on CD3 TK+ lymphocytes are shown. N = 6 or more per group from 10 independent experiments with 10 independent donors. **P < .01, versus all other samples. (E) Expression of CD27 and CD28 was assessed on TK+ lymphocytes at day 10 by flow cytometry. Relative distribution plus or minus SD of CD27+CD28+ (▨), CD27+CD28− (▩), CD27−CD28+ (▥), and CD27−CD28− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 or more per group from 9 independent experiments with 9 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2. (F) At day 10, TK+ lymphocytes were stimulated by PMA/ionomycin and analyzed by flow cytometry for IFNγ and IL-2 production. Averages plus or minus SD of IFNγ−IL-2+ (▨), IFNγ+IL-2+ (▩), and IFNγ+IL-2− (▩) cells in CD3 TK+ lymphocytes are indicated. N = 5 per group from 5 independent experiments with 5 independent donors. *P < .05; **P < .01 versus aCD3+ IL-2.
Gene-modified lymphocytes obtained after CD28 costimulation and γ-chain cytokines coexpress CD25 and CD127
IL-2, IL-7, and IL-15 play critical and coordinated roles in memory T-cell generation through signaling on specific cognate receptors, whose expressions are tightly regulated over time.33 The expression of the α subunit of the IL-7 receptor (IL-7Rα or CD127) has been shown to associate with long-term persistence of tumor-reactive CD8+ T lymphocytes in vivo.34 At different times after genetic modification, we analyzed TK+ lymphocytes for CD127 and CD25 (IL-2Rα) expression. At day 4, the majority of CD4+ and CD8+TK+ lymphocytes, regardless of memory functional phenotype and type of stimulation used for generation, gained CD25 and lost CD127 expression (Figure 2A,B). At later times, a significant proportion of CD4+ and CD8+TK+ lymphocytes generated with aCD3+ IL-2 down-regulated CD25 expression and failed to up-regulate CD127. On the contrary, TK+ lymphocytes generated with CD28 costimulation and cytokines, and enriched for TCM cells, homogenously maintained CD25 and acquired CD127 (Figure 2A,B).
Differential expression of γ-chain cytokine receptors during T-cell gene transfer. Kinetics of CD25 (A) and CD127 (B) expression on transduced cells is shown. Briefly, PBMCs were stimulated with either baCD3/CD28+ IL-7/IL-15 (◆), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), or aCD3+ IL-2 (□). At 48 and 72 hours after stimulation, cells were transduced with the RV SFCMM-3 Mut2. At day 7, transgene-positive cells were selected magnetically and cultured for additional 6 days with corresponding cytokines. Expression of IL-2Rα (CD25) and IL-7Rα (CD127) were measured by flow cytometry at days 2, 4, 9, and 13. Six hours before staining with anti-CD25 and anti-CD127 antibodies, cytokines were removed. Averages plus or minus SD of 6 independent experiments from different donors are shown. *P < .05; **P < .01. Asterisks only on aCD3+ IL-2 indicate significant differences versus all other samples.
), or aCD3+ IL-2 (□). At 48 and 72 hours after stimulation, cells were transduced with the RV SFCMM-3 Mut2. At day 7, transgene-positive cells were selected magnetically and cultured for additional 6 days with corresponding cytokines. Expression of IL-2Rα (CD25) and IL-7Rα (CD127) were measured by flow cytometry at days 2, 4, 9, and 13. Six hours before staining with anti-CD25 and anti-CD127 antibodies, cytokines were removed. Averages plus or minus SD of 6 independent experiments from different donors are shown. *P < .05; **P < .01. Asterisks only on aCD3+ IL-2 indicate significant differences versus all other samples.
Differential expression of γ-chain cytokine receptors during T-cell gene transfer. Kinetics of CD25 (A) and CD127 (B) expression on transduced cells is shown. Briefly, PBMCs were stimulated with either baCD3/CD28+ IL-7/IL-15 (◆), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), or aCD3+ IL-2 (□). At 48 and 72 hours after stimulation, cells were transduced with the RV SFCMM-3 Mut2. At day 7, transgene-positive cells were selected magnetically and cultured for additional 6 days with corresponding cytokines. Expression of IL-2Rα (CD25) and IL-7Rα (CD127) were measured by flow cytometry at days 2, 4, 9, and 13. Six hours before staining with anti-CD25 and anti-CD127 antibodies, cytokines were removed. Averages plus or minus SD of 6 independent experiments from different donors are shown. *P < .05; **P < .01. Asterisks only on aCD3+ IL-2 indicate significant differences versus all other samples.
), or aCD3+ IL-2 (□). At 48 and 72 hours after stimulation, cells were transduced with the RV SFCMM-3 Mut2. At day 7, transgene-positive cells were selected magnetically and cultured for additional 6 days with corresponding cytokines. Expression of IL-2Rα (CD25) and IL-7Rα (CD127) were measured by flow cytometry at days 2, 4, 9, and 13. Six hours before staining with anti-CD25 and anti-CD127 antibodies, cytokines were removed. Averages plus or minus SD of 6 independent experiments from different donors are shown. *P < .05; **P < .01. Asterisks only on aCD3+ IL-2 indicate significant differences versus all other samples.
Since depletion of regulatory T lymphocytes prior to polyclonal T-cell stimulation has been shown to partially preserve the immune function of T lymphocytes designed for cell therapy,35 we investigated whether natural regulatory T cells (Tregs) were preferentially expanded during our cultures. Although TCMTK+ cells maintained CD127 expression long term, arguing against a Treg function, approximately 40% of TEMTK+ cells obtained after CD3 stimulation and culture with IL-2 lost CD127 expression in 14 days of culture. In this TK+ cell population, the mean proportion of CD4+/CD25+/foxp3+ cells was not different from that of unmanipulated lymphocytes from the same donors (5.8% ± 2.4% vs 5.0% ± 1.9%, average results from 6 different donors), suggesting that natural Tregs are not expanded upon OKT3+ IL-2 activation.
Gene-modified lymphocytes expanded with IL-7 and IL-15 proliferate in response to alloantigen, self-renew, and resist activation-induced cell death
To study the alloreactive potential of TK+ lymphocytes, we labeled the cells with CFSE and we challenged them with irradiated allogeneic PBMCs in the absence of cytokines. As control responding cells, we used unmanipulated peripheral blood CD3 lymphocytes. A significantly higher proportion of baCD3/CD28+ IL-7/IL-15–cultured CD8+ cells, enriched for TCM lymphocytes, diluted the dye in response to both primary and secondary alloantigen stimulation, compared with aCD3+ IL-2–cultured CD8+ cells, enriched for TEM lymphocytes (Figure 3A). TCM-enriched CD4+TK+ cells were generally more alloreactive than TEM-enriched CD4+TK+ lymphocytes, although statistical significance was reached only for baCD3/CD28+ IL-7 TK+ lymphocytes (Figure 3A).
IL-7 and IL-15 preserve high proliferative potential, self-renewal ability, and low sensitivity to activation-induced cell death in gene-modified TCM CD8 and CD4 lymphocytes. Immune-selected TK+ lymphocytes harvested at day 10 and unmanipulated CD3+ lymphocytes (PBLs) from the same donor were labeled by CFSE, plated at the concentration of 106/mL, and cocultured with irradiated allogeneic PBMCs at a 1:1 ratio for 7 days. Viable cells were then harvested and restimulated by the same conditions for additional 7 days. No cytokine was added. 1st indicates first allostimulation; 2nd, second allostimulation. (A) Number of viable CD8+ and CD4+ lymphocytes that had proliferated, as measured by CFSE dilution (CFSEdim), 6 days after each stimulation, were calculated according to the formula: (no. of viable cells × % of CFSEdim cells). baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). N = 7 or more per group from 4 independent experiments with 9 independent donors. Allo Ag indicates cells stimulated with allogeneic irradiated PBMCs; no Ag, mock-stimulated cells. Nonparametric analysis was performed. *P < .05; **P < .01. (B) CCR7 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE content (x-axis) versus CCR7 expression (y-axis) on CD3+ lymphocytes. Results from 1 representative of 5 independent experiments with 10 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CCR7-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). N = 7 or more per group from 4 independent experiments with 9 independent donors. Allo Ag indicates cells stimulated with allogeneic irradiated PBMCs; no Ag, mock-stimulated cells. Nonparametric analysis was performed. *P < .05; **P < .01. (B) CCR7 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE content (x-axis) versus CCR7 expression (y-axis) on CD3+ lymphocytes. Results from 1 representative of 5 independent experiments with 10 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CCR7-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Results of 5 independent experiments with 10 independent donors are shown. *P < .05; **P < .01. Asterisks only on a bar indicate significant differences versus aCD3+ IL-2. (C) CD127 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE staining (x-axis) versus staining with CD127 (y-axis) in gated CD3+ lymphocytes after 1 and 2 stimulation cycles. Results from 1 representative of 6 independent experiments with 11 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CD127-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). Results of 5 independent experiments with 10 independent donors are shown. *P < .05; **P < .01. Asterisks only on a bar indicate significant differences versus aCD3+ IL-2. (C) CD127 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE staining (x-axis) versus staining with CD127 (y-axis) in gated CD3+ lymphocytes after 1 and 2 stimulation cycles. Results from 1 representative of 6 independent experiments with 11 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CD127-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Results of 6 independent experiments with 11 independent donors are shown. *P < .05; **P < .01, versus aCD3+ IL-2. (D) Activation-induced cell death was measured on CFSE-stained TK+ lymphocytes and PBLs after stimulation with escalating OKT3 concentrations. The percentage of To-Pro-3+ cells in CFSEdim T lymphocytes was measured by flow cytometry. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). Results of 6 independent experiments with 11 independent donors are shown. *P < .05; **P < .01, versus aCD3+ IL-2. (D) Activation-induced cell death was measured on CFSE-stained TK+ lymphocytes and PBLs after stimulation with escalating OKT3 concentrations. The percentage of To-Pro-3+ cells in CFSEdim T lymphocytes was measured by flow cytometry. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). N = 3 per group from 2 independent experiments with 3 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2.
). N = 3 per group from 2 independent experiments with 3 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2.
IL-7 and IL-15 preserve high proliferative potential, self-renewal ability, and low sensitivity to activation-induced cell death in gene-modified TCM CD8 and CD4 lymphocytes. Immune-selected TK+ lymphocytes harvested at day 10 and unmanipulated CD3+ lymphocytes (PBLs) from the same donor were labeled by CFSE, plated at the concentration of 106/mL, and cocultured with irradiated allogeneic PBMCs at a 1:1 ratio for 7 days. Viable cells were then harvested and restimulated by the same conditions for additional 7 days. No cytokine was added. 1st indicates first allostimulation; 2nd, second allostimulation. (A) Number of viable CD8+ and CD4+ lymphocytes that had proliferated, as measured by CFSE dilution (CFSEdim), 6 days after each stimulation, were calculated according to the formula: (no. of viable cells × % of CFSEdim cells). baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). N = 7 or more per group from 4 independent experiments with 9 independent donors. Allo Ag indicates cells stimulated with allogeneic irradiated PBMCs; no Ag, mock-stimulated cells. Nonparametric analysis was performed. *P < .05; **P < .01. (B) CCR7 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE content (x-axis) versus CCR7 expression (y-axis) on CD3+ lymphocytes. Results from 1 representative of 5 independent experiments with 10 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CCR7-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). N = 7 or more per group from 4 independent experiments with 9 independent donors. Allo Ag indicates cells stimulated with allogeneic irradiated PBMCs; no Ag, mock-stimulated cells. Nonparametric analysis was performed. *P < .05; **P < .01. (B) CCR7 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE content (x-axis) versus CCR7 expression (y-axis) on CD3+ lymphocytes. Results from 1 representative of 5 independent experiments with 10 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CCR7-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Results of 5 independent experiments with 10 independent donors are shown. *P < .05; **P < .01. Asterisks only on a bar indicate significant differences versus aCD3+ IL-2. (C) CD127 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE staining (x-axis) versus staining with CD127 (y-axis) in gated CD3+ lymphocytes after 1 and 2 stimulation cycles. Results from 1 representative of 6 independent experiments with 11 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CD127-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). Results of 5 independent experiments with 10 independent donors are shown. *P < .05; **P < .01. Asterisks only on a bar indicate significant differences versus aCD3+ IL-2. (C) CD127 expression was measured on CFSE-stained TK+ lymphocytes and PBLs 6 days after first and second allogeneic stimulation. Contour plots show CFSE staining (x-axis) versus staining with CD127 (y-axis) in gated CD3+ lymphocytes after 1 and 2 stimulation cycles. Results from 1 representative of 6 independent experiments with 11 independent donors are shown. Histograms show averages plus or minus SD of the percentage of CD127-expressing cells gated in CFSEdim CD3+ lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Results of 6 independent experiments with 11 independent donors are shown. *P < .05; **P < .01, versus aCD3+ IL-2. (D) Activation-induced cell death was measured on CFSE-stained TK+ lymphocytes and PBLs after stimulation with escalating OKT3 concentrations. The percentage of To-Pro-3+ cells in CFSEdim T lymphocytes was measured by flow cytometry. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). Results of 6 independent experiments with 11 independent donors are shown. *P < .05; **P < .01, versus aCD3+ IL-2. (D) Activation-induced cell death was measured on CFSE-stained TK+ lymphocytes and PBLs after stimulation with escalating OKT3 concentrations. The percentage of To-Pro-3+ cells in CFSEdim T lymphocytes was measured by flow cytometry. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). N = 3 per group from 2 independent experiments with 3 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2.
). N = 3 per group from 2 independent experiments with 3 independent donors. *P < .05; **P < .01, versus aCD3+ IL-2.
Long-term immunity requires that gene-modified memory lymphocytes maintain the ability to self-renew. To investigate TK+ lymphocyte self-renewal, we analyzed CCR7 expression on TK+ lymphocytes that proliferated in response to alloantigen. As shown in Figure 3B, a sizeable proportion of responding TCMTK+ lymphocytes retained the expression of CCR7. In parallel, we analyzed IL-7Rα and found that whereas responding TK+ lymphocytes obtained with CD28 costimulation maintained CD127 expression, responding TK+ lymphocytes obtained with CD3 stimulation alone down-regulated CD127 (all conditions with baCD3/CD28 versus aCD3+ IL-2, P < .01).
Long-term persistence of TCM cells has also been linked to their ability to resist AICD. To test this, we challenged CFSE-labeled peripheral blood lymphocytes (PBLs) or TK+ lymphocytes with increasing amounts of plastic-bound anti-CD3 antibody and evaluated their survival. As shown in Figure 3D, even low amounts of anti-CD3 antibodies prompted the apoptosis of TEM-enriched TK+ lymphocytes. On the contrary, increasing amounts of anti-CD3 antibodies failed to trigger AICD in TCM-enriched TK+ lymphocytes. At the highest dose, only TK+ lymphocytes generated with baCD3/CD28 and a combination of IL-7 and IL-15 resisted AICD significantly more than TK+ lymphocytes obtained in the absence of costimulation (P < .05).
Gene-modified lymphocytes cultured with IL-7 and IL-15 are highly alloreactive in a fully humanized animal model of GVHD
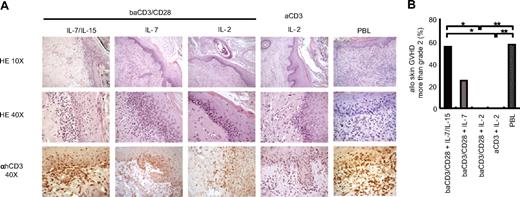
Genetic modification of human T lymphocytes reduces T-cell alloreactivity. This has been linked to the fact that current clinical protocols for genetic modification are biased toward TEM cells.18 To demonstrate that suicide gene–modified TCM cells retain considerable alloreactivity, we set up a novel, fully humanized animal model of GVHD. Immunodeficient NOD/Scid mice received a transplant of full-thickness human skin and, after conditioning with irradiation and anti–NK-cell antibodies, we infused the skin transplant recipients with mismatched unmanipulated human peripheral blood lymphocytes (PBLs) or with TK+ lymphocytes. In this model, unmanipulated human allogeneic T lymphocytes reached a median of 74% of circulating leukocytes 2 weeks after infusion (Figure 4A) and caused skin GVHD in 6 (85%) of 7 animals, with severe GVHD in 4 (57%) of 7 animals (Figure 5B). Skin GVHD in this model was characterized by a variable degree of spongiosis, diffuse basal cell vacuolization, single-cell necrosis in keratinocytes, satellitosis, lymphocytic exocytosis, and dermal melanophages (Figure 5A top and middle panels). Altogether, these findings were diagnostic for human GVHD, as observed in the clinic. The specificity of the pathological changes on human skin grafts was further confirmed by immunohistochemical staining for human CD3 (Figure 5A bottom panel). When TK+ lymphocytes were infused instead of unselected PBLs, we observed comparable levels of circulating human T lymphocytes (median 79%) only in the case of TK+ TCM-enriched lymphocytes cultured with IL-7 and IL-15 (Figure 4A). Both CD4+ and CD8+ lymphocytes persisted in vivo, with a predominance of CD4+ lymphocytes in the case of TK+ lymphocytes generated with CD28 costimulation (Figure 4B). Of interest, when we analyzed the memory phenotype of circulating human T lymphocytes, we found that, as for unmanipulated PBLs, TK+ lymphocytes cultured with IL-7 and IL-15 were giving rise to a mixed population of TK+ TEM and TCM cells (Figure 4C) that retained, to some extent, IL-7Rα expression (Figure 4D). This was associated with a high incidence of severe skin GVHD (Figure 5A,B), which was not different from that caused by unmanipulated PBLs. The infusion of TK+ TEM cells was responsible of scattered, small lymphocytic infiltrates, generally unable to exceed the basal skin layer (Figure 5A top and middle panels). Again, the specificity of the pathological changes caused by TK+ lymphocytes on human skin grafts was confirmed by immunohistochemical staining for human CD3 (Figure 5A bottom panels). Central memory TK+ lymphocytes generated with baCD3/CD28 and IL-7/IL-15 were as efficient as unmanipulated lymphocytes, more alloreactive than TEM gene-modified lymphocytes, and strikingly, more alloreactive than baCD3/CD28+ IL-2 TK+ lymphocytes, as shown by a higher incidence of severe GVHD (Figure 5B). These results confirm that IL-7 and IL-15 preserve both self-renewal potential and the ability of TCM gene–modified lymphocytes to differentiate into efficient effectors.
Engraftment and expansion of TK+ lymphocytes in NOD/Scid mice that received a transplant of allogeneic human skin. NOD/Scid mice received a transplant of human skin (day 0), and were conditioned with anti-NK antibodies (day 10) and sublethal irradiation (day 11) prior to the intravenous infusion of TK+ lymphocytes or PBLs (day 11). TK+ lymphocytes infused to mice were transduced with optimized Mut2-SFCMM3 vector, enabling a transduction efficiency higher than 30% in all conditions. (A) Kinetics of human chimerism calculated as [% of circulating human CD3+ lymphocytes]/[(% of circulating murine CD45+ cells) + (% of circulating human CD3+ lymphocytes)] are shown. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Medians of 8 independent experiments with 13 different donor cells are shown. *P < .05; **P < .01. WBC indicates white blood cells. (B) At weeks 1 and 2 after lymphocyte infusion, CD4/CD8 ratio was analyzed on circulating human CD3 lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). Medians of 8 independent experiments with 13 different donor cells are shown. *P < .05; **P < .01. WBC indicates white blood cells. (B) At weeks 1 and 2 after lymphocyte infusion, CD4/CD8 ratio was analyzed on circulating human CD3 lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Averages plus or minus SD of 6 independent experiments with 11 different donor cells are shown. *P < .05. (C) At weeks 1 and 2 after lymphocyte infusion, human CD3+ lymphocytes were analyzed by flow cytometry for CD45RA and CD62L expression. Percentages of circulating human TCM (CD45RA−CD62L+) and TEM (CD45RA−CD62L−) T lymphocytes were measured in mice that received a transplant of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes (
). Averages plus or minus SD of 6 independent experiments with 11 different donor cells are shown. *P < .05. (C) At weeks 1 and 2 after lymphocyte infusion, human CD3+ lymphocytes were analyzed by flow cytometry for CD45RA and CD62L expression. Percentages of circulating human TCM (CD45RA−CD62L+) and TEM (CD45RA−CD62L−) T lymphocytes were measured in mice that received a transplant of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes ( ), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line). Medians of 8 independent experiments with 13 different donors are shown. *P < .05; **P < .01. Asterisks on aCD3+ IL-2 indicate significant differences versus all the other samples. (D) At weeks 1 and 2 after lymphocyte infusion, IL-7Rα expression was assessed on circulating human CD3+ lymphocytes. Percentages CD3+ IL-7Rα+ T lymphocytes on animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes (
), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line). Medians of 8 independent experiments with 13 different donors are shown. *P < .05; **P < .01. Asterisks on aCD3+ IL-2 indicate significant differences versus all the other samples. (D) At weeks 1 and 2 after lymphocyte infusion, IL-7Rα expression was assessed on circulating human CD3+ lymphocytes. Percentages CD3+ IL-7Rα+ T lymphocytes on animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes ( ), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line) were measured. Medians of 8 experiments with 13 independent donors are shown. *P < .05; **P < .01 versus aCD3+ IL-2.
), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line) were measured. Medians of 8 experiments with 13 independent donors are shown. *P < .05; **P < .01 versus aCD3+ IL-2.
Engraftment and expansion of TK+ lymphocytes in NOD/Scid mice that received a transplant of allogeneic human skin. NOD/Scid mice received a transplant of human skin (day 0), and were conditioned with anti-NK antibodies (day 10) and sublethal irradiation (day 11) prior to the intravenous infusion of TK+ lymphocytes or PBLs (day 11). TK+ lymphocytes infused to mice were transduced with optimized Mut2-SFCMM3 vector, enabling a transduction efficiency higher than 30% in all conditions. (A) Kinetics of human chimerism calculated as [% of circulating human CD3+ lymphocytes]/[(% of circulating murine CD45+ cells) + (% of circulating human CD3+ lymphocytes)] are shown. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Medians of 8 independent experiments with 13 different donor cells are shown. *P < .05; **P < .01. WBC indicates white blood cells. (B) At weeks 1 and 2 after lymphocyte infusion, CD4/CD8 ratio was analyzed on circulating human CD3 lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (
). Medians of 8 independent experiments with 13 different donor cells are shown. *P < .05; **P < .01. WBC indicates white blood cells. (B) At weeks 1 and 2 after lymphocyte infusion, CD4/CD8 ratio was analyzed on circulating human CD3 lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 ( ), baCD3/CD28+ IL-2 (
), baCD3/CD28+ IL-2 ( ), aCD3+ IL-2 (□), or PBLs (
), aCD3+ IL-2 (□), or PBLs ( ). Averages plus or minus SD of 6 independent experiments with 11 different donor cells are shown. *P < .05. (C) At weeks 1 and 2 after lymphocyte infusion, human CD3+ lymphocytes were analyzed by flow cytometry for CD45RA and CD62L expression. Percentages of circulating human TCM (CD45RA−CD62L+) and TEM (CD45RA−CD62L−) T lymphocytes were measured in mice that received a transplant of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes (
). Averages plus or minus SD of 6 independent experiments with 11 different donor cells are shown. *P < .05. (C) At weeks 1 and 2 after lymphocyte infusion, human CD3+ lymphocytes were analyzed by flow cytometry for CD45RA and CD62L expression. Percentages of circulating human TCM (CD45RA−CD62L+) and TEM (CD45RA−CD62L−) T lymphocytes were measured in mice that received a transplant of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes ( ), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line). Medians of 8 independent experiments with 13 different donors are shown. *P < .05; **P < .01. Asterisks on aCD3+ IL-2 indicate significant differences versus all the other samples. (D) At weeks 1 and 2 after lymphocyte infusion, IL-7Rα expression was assessed on circulating human CD3+ lymphocytes. Percentages CD3+ IL-7Rα+ T lymphocytes on animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes (
), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line). Medians of 8 independent experiments with 13 different donors are shown. *P < .05; **P < .01. Asterisks on aCD3+ IL-2 indicate significant differences versus all the other samples. (D) At weeks 1 and 2 after lymphocyte infusion, IL-7Rα expression was assessed on circulating human CD3+ lymphocytes. Percentages CD3+ IL-7Rα+ T lymphocytes on animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes ( ), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line) were measured. Medians of 8 experiments with 13 independent donors are shown. *P < .05; **P < .01 versus aCD3+ IL-2.
), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line) were measured. Medians of 8 experiments with 13 independent donors are shown. *P < .05; **P < .01 versus aCD3+ IL-2.
Central memory gene-modified lymphocytes cultured with IL-7/IL-15 are highly alloreactive. NOD/Scid mice that received a transplant of human skin, and were conditioned with anti-NK antibodies and sublethal irradiation were infused intravenously with TK+ lymphocytes or PBLs. (A) Two to 3 weeks after infusion, transplanted human skins were excised from killed mice and analyzed after staining with hematoxylin and eosin (×10 top low, ×40 middle low). Samples were simultaneously analyzed by immunohistochemistry after counterstaining with monoclonal anti-hCD3 antibodies and peroxidase-conjugated second-step reagent (×40 bottom low). Sections from representative animals of 7 independent experiments are shown. (B) Pathological grading of transplanted human skin was performed on the basis of criteria currently used for pathological diagnosis of skin GVHD in patients who underwent transplantation. Frequency of severe skin GVHD (more than grade 2) in 7 independent experiments with 9 independent T-cell donors is shown; baCD3/CD28+ IL-7/IL-15 (■, n = 9), baCD3/CD28+ IL-7 ( , n = 4), baCD3/CD28+ IL-2 (
, n = 4), baCD3/CD28+ IL-2 ( , n = 7), aCD3+ IL-2 (□, n = 5), or PBLs (
, n = 7), aCD3+ IL-2 (□, n = 5), or PBLs ( , n = 7). *P < .05; **P < .01.
, n = 7). *P < .05; **P < .01.
Central memory gene-modified lymphocytes cultured with IL-7/IL-15 are highly alloreactive. NOD/Scid mice that received a transplant of human skin, and were conditioned with anti-NK antibodies and sublethal irradiation were infused intravenously with TK+ lymphocytes or PBLs. (A) Two to 3 weeks after infusion, transplanted human skins were excised from killed mice and analyzed after staining with hematoxylin and eosin (×10 top low, ×40 middle low). Samples were simultaneously analyzed by immunohistochemistry after counterstaining with monoclonal anti-hCD3 antibodies and peroxidase-conjugated second-step reagent (×40 bottom low). Sections from representative animals of 7 independent experiments are shown. (B) Pathological grading of transplanted human skin was performed on the basis of criteria currently used for pathological diagnosis of skin GVHD in patients who underwent transplantation. Frequency of severe skin GVHD (more than grade 2) in 7 independent experiments with 9 independent T-cell donors is shown; baCD3/CD28+ IL-7/IL-15 (■, n = 9), baCD3/CD28+ IL-7 ( , n = 4), baCD3/CD28+ IL-2 (
, n = 4), baCD3/CD28+ IL-2 ( , n = 7), aCD3+ IL-2 (□, n = 5), or PBLs (
, n = 7), aCD3+ IL-2 (□, n = 5), or PBLs ( , n = 7). *P < .05; **P < .01.
, n = 7). *P < .05; **P < .01.
Activation of the suicide gene machinery in TK+ lymphocytes cultured with IL-7 and IL-15 abrogates GVHD
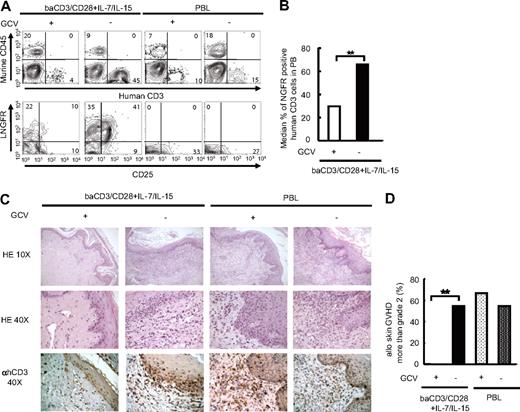
The purpose of suicide gene therapy in allo-HCT is to exploit the alloreactive response for GVL being able to block its progression to severe, life-threatening GVHD. To verify whether GVHD caused by highly alloreactive TK+ TCM cells in this model could be halted through the activation of the suicide gene machinery, we treated animals grafted with human skin and infused with fully mismatched unmanipulated PBLs or TK+ lymphocytes, with the prodrug GCV, or with saline. The chemicals were delivered 3 to 6 days after T-cell infusions through the subcutaneous implantation of an osmotic pump. In animals infused with TCMTK+ lymphocytes, treatment with GCV for 7 days was associated with a significant decrease in the level of circulating human T lymphocytes (Figure 6A,B). GCV-induced cell death of TK+ lymphocytes is cell cycle–dependent. Accordingly, the few circulating TK+ cells that survived GCV treatment were resting lymphocytes,36 as indicated by the absence of CD25 expression (Figure 6A). Most importantly, at the end of GCV treatment, no signs of severe GVHD were observed in human skin harvested from mice treated with TCMTK+ lymphocytes and rescued with GCV, indicating efficient modulation of alloreactivity by the suicide/prodrug machinery (Figure 6C,D). No effect of GCV was observed in mice infused with unmanipulated lymphocytes.
GCV administration controls GvHD-induced by TCM suicide gene–modified human lymphocytes. NOD/Scid mice received a transplant of human skin, and were conditioned with anti-NK antibodies and sublethal irradiation prior to the intravenous infusion of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes or PBLs. Three to 6 days after injection, mice received a transplant of a 5 mg GCV or PBS-filled mini-osmotic pump. (A) After 7 days of treatment with or without GCV, circulating cells were analyzed by flow cytometry after staining with antihuman CD3 (x-axis in top panel), antimurine CD45 (y-axis in top panel), antihuman CD25 (x-axis in bottom panel), and antihuman LNGFR (y-axis in bottom panel) antibodies. Results from 1 representative of 3 independent experiments are shown. (B) At day 7 of treatment, the percentage of circulating TK+ lymphocytes in mice infused with baCD3/CD28+ IL-7/IL-15 was measured by flow cytometry. Median results of 3 independent experiments with 8 independent T-cell donors are shown. baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes from mice treated with GCV (□, n = 4); baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes from mice treated without GCV (■, n = 9). **P < .01. (C) At day 7 of treatment, transplanted human skin was excised from killed mice and analyzed by histopathology after staining with hematoxylin and eosin (×10 top low, ×40 middle low). Samples were simultaneously analyzed by immunohistochemistry after counterstaining with monoclonal anti-hCD3 antibodies and peroxidase-conjugated second-step reagent (×40 bottom low). Sections from 1 representative of 3 independent experiments are shown. (D) Pathological grading of transplanted human skin was performed on the basis of criteria currently used for pathological diagnosis of skin GVHD in patients who underwent transplantation. Frequency of severe skin GVHD (more than grade 2) in animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes or PBLs, and treated with or without GCV of 3 independent experiments with 8 independent T-cell donors are shown; baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes with GCV treatment (□, n = 9), baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes without GCV treatment (■, n = 11), PBLs with GCV treatment ( , n = 3), and PBLs without GCV treatment (
, n = 3), and PBLs without GCV treatment ( , n = 11). **P < .01.
, n = 11). **P < .01.
GCV administration controls GvHD-induced by TCM suicide gene–modified human lymphocytes. NOD/Scid mice received a transplant of human skin, and were conditioned with anti-NK antibodies and sublethal irradiation prior to the intravenous infusion of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes or PBLs. Three to 6 days after injection, mice received a transplant of a 5 mg GCV or PBS-filled mini-osmotic pump. (A) After 7 days of treatment with or without GCV, circulating cells were analyzed by flow cytometry after staining with antihuman CD3 (x-axis in top panel), antimurine CD45 (y-axis in top panel), antihuman CD25 (x-axis in bottom panel), and antihuman LNGFR (y-axis in bottom panel) antibodies. Results from 1 representative of 3 independent experiments are shown. (B) At day 7 of treatment, the percentage of circulating TK+ lymphocytes in mice infused with baCD3/CD28+ IL-7/IL-15 was measured by flow cytometry. Median results of 3 independent experiments with 8 independent T-cell donors are shown. baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes from mice treated with GCV (□, n = 4); baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes from mice treated without GCV (■, n = 9). **P < .01. (C) At day 7 of treatment, transplanted human skin was excised from killed mice and analyzed by histopathology after staining with hematoxylin and eosin (×10 top low, ×40 middle low). Samples were simultaneously analyzed by immunohistochemistry after counterstaining with monoclonal anti-hCD3 antibodies and peroxidase-conjugated second-step reagent (×40 bottom low). Sections from 1 representative of 3 independent experiments are shown. (D) Pathological grading of transplanted human skin was performed on the basis of criteria currently used for pathological diagnosis of skin GVHD in patients who underwent transplantation. Frequency of severe skin GVHD (more than grade 2) in animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes or PBLs, and treated with or without GCV of 3 independent experiments with 8 independent T-cell donors are shown; baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes with GCV treatment (□, n = 9), baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes without GCV treatment (■, n = 11), PBLs with GCV treatment ( , n = 3), and PBLs without GCV treatment (
, n = 3), and PBLs without GCV treatment ( , n = 11). **P < .01.
, n = 11). **P < .01.
Discussion
Upon antigen encounter, naive T lymphocytes undergo a dynamic process of proliferation and differentiation, giving rise to effectors programmed to die as the antigen is cleared. During the process, a fraction of lymphocytes acquires peculiar functional properties ensuring long-term persistence and the ability to differentiate into effectors upon antigen re-encounter. The establishment of immunologic memory can provide life-long protection from disease recurrence.4 The adoptive transfer of tumor-reactive lymphocytes can produce significant clinical results in cancer patients, although responses are often transient.1,37 This is possibly because tumor-reactive T lymphocytes are selected for effector T cells, which, in the absence of memory lymphocytes, do not persist long term. In the case of allo-HCT, the infusion of donor T lymphocytes can achieve long-term remissions, approaching cure from leukemia. In this context, infused donor T lymphocytes consist of a mixed population of naive, memory, and effector cells, with a wide range of antigen specificities, including alloreactive lymphocytes. Unfortunately, clinical benefit is limited by the occurrence of GVHD.
Genetic modification of T lymphocytes to convey an inducible suicide program is a current clinical strategy to control GVHD. RV gene transfer requires T-cell receptor triggering and generates modified memory T lymphocytes. Clinical trials showed that gene-modified TEM cells are characterized by low alloreactive potential.19,20 The analysis of TK+ lymphocytes, harvested from treated patients, showed that TEM gene-modified cells infused into patients fail in re-expressing CD62L or CCR7. Cells maintain a CD45RA−CCR7− TEM phenotype, or progress to a CD45RA+CCR7− effector phenotype,38 in line with a linear progression of T-cell differentiation. Several animal studies substantiated the concept that TNA cells are the primary source of alloreactive lymphocytes causing GVHD.13,39,40 Several hypotheses may explain this finding: The superior access of TNA lymphocytes to secondary lymphoid organs may facilitate GVHD initiation, a hypothesis recently challenged by Anderson et al.41 Alternative explanations may be a higher frequency of alloreactive precursors in the naive T-cell compartment, and/or the higher expansion potential of TNA lymphocytes compared with memory and effector cells.42 In humans, the picture is less clear since TCM cells possibly retain substantial alloreactivity.43 Increasing the alloreactive potential of gene-modified T lymphocytes is expected to improve the efficacy of the suicide gene therapy approach to allo-HCT for the treatment of cancer. Based on this hypothesis, we generated different memory subsets of suicide gene–modified lymphocytes and comparatively studied their alloreactivity in vitro and in vivo.
In a previous study, we demonstrated that genetic modification of human T lymphocytes after CD28 costimulation on clinical-grade cell-sized beads generates highly TCM-enriched TK+ lymphocytes that, after in vitro expansion with low-dose IL-2, cause more GVHD than TEM cells in a xenograft model.21 In this model, albeit superior to TK+ TEM cells, TCM-enriched lymphocytes caused less GVHD than unmanipulated PBLs. In the current study, this was confirmed in a fully humanized animal model of GVHD. The reason may be that gene-modified lymphocytes do not contain TNA cells. On the other hand, proper CD28 costimulation is anticipated to recruit into cell cycle also TNA cells, thus enabling retroviral gene targeting to this T-lymphocyte subset. Accordingly, we observed that CD28 costimulation permits efficient (50%) gene transfer into naive lymphocytes sorted for CD45RA and CD62L expression (data not shown). This mechanism may promote preservation of a wide T-cell repertoire, including alloreactive T cells at the frequency required to induce GVHD, in TK lymphocytes. Nevertheless, we cannot exclude that differentiation of TNA lymphocytes in TCM cells may have reduced their expansion potential. The use of lentiviral vectors, able to transduce naive lymphocytes without inducing differentiation,31 will allow to overcome this possible limitation, and to further exploit alloreactivity.
An alternative explanation is that, after generation, TCM cells have peculiar cytokine requirements for the preservation of alloreactivity. Accordingly, we found that TCM-enriched TK+ lymphocytes obtained after CD28 costimulation and expanded with a combination of low-dose IL-7 and IL-15 do not differ from unmanipulated PBLs in causing GVHD in a fully humanized animal model.
Existing models of GVHD based on the infusions of T lymphocytes in immunodeficient mice assume that xenoreactivity is a good surrogate marker for alloreactivity. This assumption may be biased by 2 factors. It is recognized that the frequency of xenoreactive is less than that of alloreactive T-cell precursors.44 Moreover, NOD/Scid mice, which are among the most commonly used strains for this type of study, have variable defects in antigen presentation. The model we set up aimed at bypassing these limitations by directly evaluating the alloreactive response against human skin grafted into NOD/Scid mice. The advantage of using full-thickness human skin is that, due to the presence of professional antigen-presenting cells, such as Langerhans cells and dermal dendritic cells, it can serve as an optimal source for alloantigen. Accordingly, we observed a rapid and robust expansion of human T cells, with a level of human chimerism, detected 1 week after infusion, one log higher than that already reported in NOD/Scid models of GVHD.21 Moreover, the preservation of human skin histologic structure allowed for evaluation of specific pathological changes induced by infiltrating lymphocytes. Indeed, this model was sensitive enough to detect pathological signs such as dermo-epidermal lesions, which are highly specific for human GVHD and currently used as criteria for disease scoring in the clinic.45
In this stringent model of GVHD, despite a homogeneous phenotype, TCM gene-modified lymphocytes cultured with different cytokine combinations proved significantly different in mediating alloreactivity. This may be possibly due to a differential distribution of gene-modified lymphocytes endowed with a regulatory activity. Naturally occurring CD4+CD25+ regulatory T lymphocytes (Tregs) inhibit GVHD in mouse models.46 Initial depletion of CD4+CD25+ cells has been shown to restore the ability of mouse T lymphocytes expanded after CD3 stimulation and culture with IL-2 to mediate alloreactivity. In humans, CD4+CD25+ Tregs do not express CD127.47 In our study, regardless of the cytokine used for expansion, human TCM-enriched lymphocytes coexpressed CD127 and CD25, although the proportion of CD4+/CD25+/foxp3+ cells in TEM-enriched TK+ cells was similar to that of unmanipulated lymphocytes, suggesting that the differential alloreactivity mediated by gene-modified cells does not rely on a differential expansion of natural Tregs.
IL-7 appears a nonredundant upstream regulator that maintains the quiescent status of IL-7R expressing naive and memory T lymphocytes,23-25 whereas IL-15 promotes survival and homeostatic T-cell proliferation.26,27 In contrast to IL-2, IL-7 and IL-15 protect lymphocytes from apoptosis, a process that is critically involved during the contraction of immune responses.48,49 IL-15 has been recently proposed as a candidate cytokine for the generation of memory stem T cells able to self-renew and differentiate into alloreactive effectors.14 When repetitively challenged with alloantigens in vitro, a large proportion of TK+ TCM cells proliferated while maintaining CCR7 or IL-7rα expression. Moreover, TCM-enriched TK+ lymphocytes expanded with IL-7 and IL-15 showed high resistance to AICD. When infused into NOD/Scid mice grafted with mismatched human skin, only TCM-enriched TK+ lymphocytes expanded with IL-7 and IL-15 engrafted and survived to levels comparable with unmanipulated PBLs. Of interest, TCM-enriched TK+ lymphocytes expanded with IL-7 and IL-15 gave rise in vivo to a mixed population of TCM and TEM cells. Altogether these results suggest that putative human alloreactive memory stem T cells are contained in the CD45RA−CCR7+ IL-7Rα+ subset, and may be preserved after genetic modification by exposure to IL-7 and IL-15.
In this fully mismatched GVHD model, early treatment of GVHD by ganciclovir induced complete disease control. Current knowledge on the pathophysiology of GVHD indicates that the activation and expansion of allogeneic donor lymphocytes, critical for GVHD initiation, trigger a cascade of inflammatory events that amplify the disease.50 The TK/ganciclovir technology aims at decapitating GVHD in the early, T cell–dependent phase, and this approach proved effective in at least 7 independent clinical trials of TK-cell gene therapy.18
The use of gene-modified lymphocytes able to self-renew and differentiate into effectors is the premise to improve efficacy of cancer T-cell therapy. However, wide expansion and long-term persistence of transferred cells requires a strict safety assessment. In our study, transfer and expression of the TK suicide gene allowed an efficient conditional-suicide of TCM lymphocytes, and control of GVHD. These results indicate that suicide gene transfer into TCM cells permits to safely exploit the potency of alloreactivity to cure cancer. In particular, the clinical use of highly alloreactive TK TCM lymphocytes in high-risk patients, such as patients affected by persistent leukemia at time of allo-HCT, will be investigated with the purpose of safely exploiting the alloreactive potential of donor lymphocytes.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Prof Tanaka (Osaka University, Osaka, Japan) for kindly providing the TMβ-1 hybridoma; Veronica Valtolina, Zulma Magnani, Martina Rocchi, and Giuseppina Marano for technical assistance; and Serena Kimi Perna and Marco Luciano Ferrari for critical discussion. This work is in memory of Salvatore Toma, who died while helping the authors with this article.
This work was supported by the Italian Ministry of Health (Rome, Italy), the Italian Ministry of Research and University (Rome, Italy), Fondazione Cariplo (Milan, Italy), and the Italian Association for Cancer Research (AIRC; Milan, Italy).
Authorship
Contribution: S.K. and A.B. designed and performed research, analyzed data, and wrote the paper; S.M. performed research and analyzed the data; M.P. and F.S. processed samples, analyzed data, and commented on the paper; L.A. provided and processed samples, and commented on the paper; M.R. provided vital reagent and commented on the paper; S.L.S.-C. and E.P. performed research; A.M. contributed to the design of research and commented on the paper; T.N. designed the in vivo model of alloreactivity and commented on the paper; K.F. designed the in vitro models of alloreactivity and commented on the paper; V.R. and F.C. contributed to design of the research and commented on the paper; C.T. designed the retroviral vector and commented on the paper; C. Bordignon supervised the research and commented on the paper; and C. Bonini designed, supervised, and performed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: F.C. and C. Bonini have declared financial interest in MolMed Spa, whose potential product was studied in the present work. M.R., S.L.S.-C., C.T., and C. Bordignon are employees of MolMed Spa, whose potential product was studied in the present work. The remaining authors declare no competing financial interests.
Correspondence: Attilio Bondanza, Experimental Hematology Unit, Cancer Immunotherapy and Gene Therapy Program, San Raffaele Scientific Institute, Via Olgettina 58, 20132 Milan, Italy; e-mail: attilio.bondanza@hsr.it.

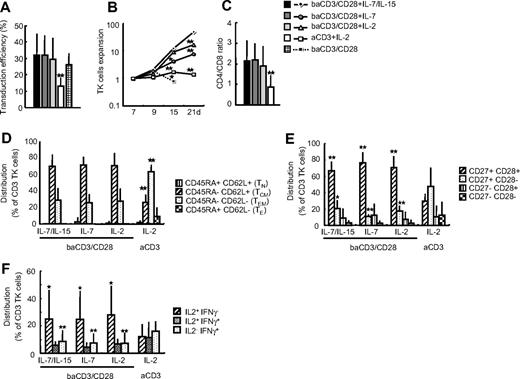

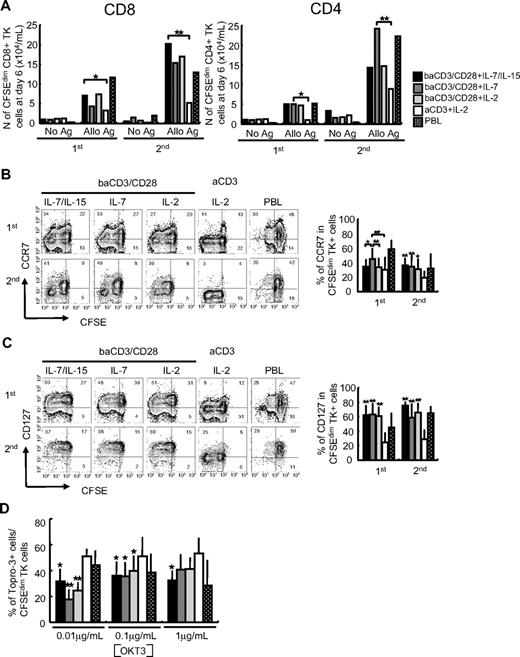
![Figure 4. Engraftment and expansion of TK+ lymphocytes in NOD/Scid mice that received a transplant of allogeneic human skin. NOD/Scid mice received a transplant of human skin (day 0), and were conditioned with anti-NK antibodies (day 10) and sublethal irradiation (day 11) prior to the intravenous infusion of TK+ lymphocytes or PBLs (day 11). TK+ lymphocytes infused to mice were transduced with optimized Mut2-SFCMM3 vector, enabling a transduction efficiency higher than 30% in all conditions. (A) Kinetics of human chimerism calculated as [% of circulating human CD3+ lymphocytes]/[(% of circulating murine CD45+ cells) + (% of circulating human CD3+ lymphocytes)] are shown. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (), baCD3/CD28+ IL-2 (), aCD3+ IL-2 (□), or PBLs (). Medians of 8 independent experiments with 13 different donor cells are shown. *P < .05; **P < .01. WBC indicates white blood cells. (B) At weeks 1 and 2 after lymphocyte infusion, CD4/CD8 ratio was analyzed on circulating human CD3 lymphocytes. baCD3/CD28+ IL-7/IL-15 (■), baCD3/CD28+ IL-7 (), baCD3/CD28+ IL-2 (), aCD3+ IL-2 (□), or PBLs (). Averages plus or minus SD of 6 independent experiments with 11 different donor cells are shown. *P < .05. (C) At weeks 1 and 2 after lymphocyte infusion, human CD3+ lymphocytes were analyzed by flow cytometry for CD45RA and CD62L expression. Percentages of circulating human TCM (CD45RA−CD62L+) and TEM (CD45RA−CD62L−) T lymphocytes were measured in mice that received a transplant of baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes (), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line). Medians of 8 independent experiments with 13 different donors are shown. *P < .05; **P < .01. Asterisks on aCD3+ IL-2 indicate significant differences versus all the other samples. (D) At weeks 1 and 2 after lymphocyte infusion, IL-7Rα expression was assessed on circulating human CD3+ lymphocytes. Percentages CD3+ IL-7Rα+ T lymphocytes on animals infused with baCD3/CD28+ IL-7/IL-15 TK+ lymphocytes (♦), baCD3/CD28+ IL-7 TK+ lymphocytes (), baCD3/CD28+ IL-2 TK+ lymphocytes (light gray triangle), aCD3+ IL-2 TK+ lymphocytes (white square with broken line), or PBLs (X with dotted line) were measured. Medians of 8 experiments with 13 independent donors are shown. *P < .05; **P < .01 versus aCD3+ IL-2.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/113/5/10.1182_blood-2008-05-156059/4/m_zh80040929960004.jpeg?Expires=1769124631&Signature=MLzUzHH5a1tzcdraYQ-bcqC2RtSxDSWK6QjqumPg8OWBKRBUFxHP1q--mMrrFBJJgQ9ynme9aBfNe8Zd8ecaBgfoS83WfbqLtNHCFAAJP1A4Yd9ogggeINeT5bPWhMr~upqYumjDncn1kxye37sg-tMCGrohqdxAlV~5tludcQBlZdzOAYi3hRXISXPnuUGbHj49oxxVPuJPN7G~eE5pYiFniAG8ckB0a-1DWE~YwCKKv4RHXqbmXX5Zzt-rdjy5gbc8~O8zi7zUax7F56uKsNn6pWJHSZRYeokQ6NwEBe5OHPDVq7qOLCRa3xB4mkkAPwCSUIAgzvtShVDPNyeZ-w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)