Abstract
Histone deacetylase (HDAC) enzymatic activity has been linked to the transcription of DNA in cancers including multiple myeloma (MM). Therefore, HDAC inhibitors used alone and in combination are being actively studied as novel therapies in MM. In the present study, we investigated the preclinical activity of ACY-1215, an HDAC6-selective inhibitor, alone and in combination with bortezomib in MM. Low doses of ACY-1215 combined with bortezomib triggered synergistic anti-MM activity, resulting in protracted endoplasmic reticulum stress and apoptosis via activation of caspase-3, caspase-8, and caspase-9 and poly (ADP) ribosome polymerase. In vivo, the anti-MM activity of ACY-1215 in combination with bortezomib was confirmed using 2 different xenograft SCID mouse models: human MM injected subcutaneously (the plasmacytoma model) and luciferase-expressing human MM injected intravenously (the disseminated MM model). Tumor growth was significantly delayed and overall survival was significantly prolonged in animals treated with the combination therapy. Pharmacokinetic data showed peak plasma levels of ACY-1215 at 4 hours after treatment coincident with an increase in acetylated α-tubulin, a marker of HDAC6 inhibition, by immunohistochemistry and Western blot analysis. These studies provide preclinical rationale for acetylated α-tubulin use as a pharmacodynamic biomarker in future clinical trials.
Introduction
Significant progress has been made in the treatment of multiple myeloma (MM) in the past decade because of the introduction of novel therapies.1,2 Proteasome inhibitors such as bortezomib represent a promising class of novel agents with marked anti-MM activity3 ; however, the rate of MM relapse remains high,4 stimulating the investigation of novel targets for combination therapies. In this context, the combination of proteasome inhibitors with histone deacetylase (HDAC) inhibitors has shown very promising results in preclinical MM models.5-9
HDACs are histone-modifying enzymes that regulate gene transcription.10 Histone acetyl transferases add acetyl groups to target histones, relaxing chromatin structure and allowing gene transcription. In contrast, HDACs remove acetyl groups from core histones, condensing DNA structure and thus preventing gene transcription.11 Changes in histone modification are commonly found in human cancers, including MM,12 making the HDACs attractive therapeutic targets, and several small-molecule HDAC inhibitors have been investigated in preclinical models of hematologic malignancies.6,13-16 Currently, HDAC inhibitors being tested in clinical trials can be divided into 2 groups: (1) nonselective pan-HDAC inhibitors such as vorinostat (SAHA) and panobinostat, which predominately target class I (HDAC1, HDAC2, and HDAC3) and class IIb (HDAC6) HDAC inhibitors; and (2) class I HDAC inhibitors such as romidepsin and entinostat, which target only class I.6,17 Preliminary data from 2 phase 1 clinical trials of bortezomib with SAHA in refractory MM patients showed significant responses even in bortezomib-resistant patients, with an overall response rate of 42%18 and 46%,19,20 prompting phase 2 and 3 studies with promising responses. Mild to moderate fatigue, prolonged QT interval, and hematologic and gastrointestinal toxicities were observed.18-20 In a phase 1b study of the other pan-HDAC inhibitor, panobinostat, in combination with bortezomib showed promising activity in relapsed and refractory MM patients, with a response rate of 62% even in bortezomib-refractory patients. The most common toxicities of these broad HDAC inhibitors are thrombocytopenia, diarrhea, and fatigue.21,22 A phase 1/2 clinical trial of romidepsin in combination with bortezomib and dexamethasone showed significant response in relapsed and refractory MM patients, with an overall response rate of 67%. No significant increase in thrombocytopenia compared with single-agent bortezomib and romidepsin was observed in the combination therapy.23
Although the mechanism of action responsible for the synergistic activity of HDAC inhibitors with bortezomib is not fully understood, one suggested mechanism is the role of HDAC6 in aggresomal degradation of ubiquitinated proteins.5 Specifically, proteasome inhibition induces the accumulation of unfolded and misfolded, ubiquitin-conjugated proteins in perinuclear aggresomes.24 HDAC6 activity plays a crucial role in the formation of perinuclear aggresomes; conversely, targeting HDAC6 with gene knock-down strategies or with the selective inhibitor tubacin enhances proteasome inhibitor activity. Targeting both proteasomal and aggresomal protein degradation systems with proteasome and HDAC6 inhibitors, respectively, induces accumulation of polyubiquitinated proteins, eliciting apoptotic cascades and synergistic cytotoxicity.5,25 These findings present HDAC6 as an interesting novel target. Moreover, inhibiting HDAC6 selectively may not only enhance potency, but may also reduce the toxicity related to off-target effects of pan-HDAC inhibitors. To date, small molecules such as tubacin and tubastatin have been developed to target HDAC65,26,27 ; however, these research probe compounds are not optimized for oral delivery and cannot be tested in clinical trials.
In the present study, we investigate the preclinical activity of ACY-1215, a novel, selective, orally bioavailable HDAC6 inhibitor, alone and in combination with bortezomib. In addition to characterizing its molecular mechanism of anti-MM activity, we define the preclinical pharmacologic, pharmacokinetic (PK), and pharmacodynamic (PD) profiles of ACY-1215 alone and in combination with bortezomib, in 2 MM xenograft mouse models. Our data inform the design of a currently accruing clinical trial evaluating ACY-1215 alone and combined with bortezomib in MM.
Methods
Cell lines and reagents
Dexamethasone (Dex)–sensitive (MM.1S) and Dex-resistant (MM.1R) human MM cell lines were provided by Dr Steven Rosen (Northwestern University, Chicago, IL). RPMI8226 and U266 human MM cells were obtained from ATCC. Melphalan-resistant RPMI-LR5 (LR5) and doxorubicin-resistant RPMI-Dox40 (Dox40) cell lines were provided by Dr William Dalton (H. Lee Moffitt Cancer Center, Tampa, FL). OPM1 cells were provided by Dr P. Leif Bergsagel (Mayo Clinic, Scottsdale, AZ). ANBL-6 bortezomib-resistant (ANBL-6.BR) cells were provided by Dr Robert Orlowski (MD Anderson Cancer Center, Houston, TX). All MM cell lines were cultured as described previously.28 Fresh PBMCs were obtained from 4 healthy volunteers. BM aspirates from MM patients were obtained after approval from the Massachusetts General Hospital Institutional Review Board. After mononuclear cell separation, MM cells were purified by positive CD138 (Syndecan-1) MicroBead selection, as described previously.29 BM stromal cells (BMSCs) were generated as described previously28 and incubated in 96-well culture plates (1 × 104 BMSCs/well) for 24 hours. After washing, MM cell lines were added to the wells (2 × 104cells/well) and incubated with medium or with increasing doses of ACY-1215 for the specified times at 37°C.
2-(Diphenylamino)-N-(7-(hydroxyamino)-7-oxoheptyl)pyrimidine-5-carboxamide (ACY-1215) was synthesized by ChemPartner and obtained from Acetylon Pharmaceuticals. ACY-1215 was dissolved first in DMSO (Sigma-Aldrich) at a concentration of 10mM, and then in culture medium (0.5-8μM) immediately before use. HDAC1, HDAC2, HDAC3, and HDAC6 were obtained from BPS Biosciences. The fluorophore tripeptide substrate (FTS) was prepared by ChemPartner. The class IIa tripeptide substrate MAZ-1675 was synthesized in the laboratory of R.M.17
Bortezomib was obtained from Selleck Chemicals for the in vitro studies. It was dissolved first in DMSO at a concentration of 20mM, and then in culture medium before use. Bortezomib for the in vivo studies was purchased from the Dana-Farber Cancer Institute pharmacy.
HDAC enzymatic assays
ACY-1215 was dissolved and subsequently diluted in assay buffer [50mM HEPES, pH 7.4, 100mM KCl, 0.001% Tween-20, 0.05% BSA, and 20μM tris(2-carboxyethyl)phosphine] to 6-fold the final concentration. HDAC enzymes were diluted to 1.5-fold of the final concentration in assay buffer and pre-incubated with ACY-1215 for 10 minutes before the addition of the substrate. The amount of FTS (HDAC1, HDAC2, HDAC3, and HDAC6) or MAZ-1675 (HDAC4, HDAC5, HDAC7, HDAC8, and HDAC9) used for each enzyme was equal to the Michaelis constant (Km), as determined by a titration curve. FTS or MAZ-1675 was diluted in assay buffer to 6-fold the final concentration with 0.3μM sequencing grade trypsin (Sigma-Aldrich). The substrate/trypsin mix was added to the enzyme/compound mix and the plate was shaken for 60 seconds and then placed into a SpectraMax M5 microtiter plate reader. The enzymatic reaction was monitored for release of 7-amino-4-methoxy-coumarin over 30 minutes, after deacetylation of the lysine side chain in the peptide substrate, and the linear rate of the reaction was calculated. HDAC11, sirtuin1, and sirtuin2 assays were performed by Cerep.
Cell viability and proliferation assays:
The effect of ACY-1215 with or without bortezomib on the viability of MM cell lines, patient MM cells, and PBMCs was assessed by measuring MTT (Chemicon International) dye absorbance, as described previously.29 PBMCs from healthy donors were isolated and stimulated with 2.5 μg/mL of phytohemagglutinin (PHA) for 48 hours in the presence of increasing concentrations of ACY-1215. DNA synthesis was measured by tritiated thymidine uptake (PerkinElmer). CD4+ T cells were purified from human blood with the Rosette Sep negative-selection kit (StemCell Technologies). Cells were stimulated by CD3/CD28 Dynabeads for 7 days in the presence of compounds. Cell viability was assessed using alamarBlue (Invitrogen). MM cells (2-3 × 104cells/well) were incubated in 96-well culture plates with medium and different concentrations of ACY-1215, bortezomib, and/or recombinant IL-6 (10 ng/mL) or insulin-like growth factor-1 (IGF-1; 50 ng/mL) for 24 hours at 37°C, and tritiated thymidine incorporation was measured, as described previously.29
Detection of apoptosis
MM cells (1 × 106) were cultured for 24 hours in medium alone or medium with various concentrations of ACY-1215 and/or bortezomib. Cells were harvested, washed, and stained with annexin V/propidium iodide (PI) as described previously.28 Annexin V+/PI− apoptotic cells were enumerated using the Epics flow cytometer. The percentage of cells undergoing apoptosis was defined as the sum of early apoptosis (annexin V+) and late apoptosis (annexin V+ and PI+) cells.
Western blotting
MM cells were cultured with different concentrations of ACY-1215 and/or bortezomib, harvested, washed, and lysed as described previously.28 Cell lysates were subjected to SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with: antiacetylated α-tubulin (Sigma-Aldrich); antiubiquitin, acetylated-lysine, caspase-3, caspase-8, and caspase-9, poly(ADP-ribose) polymerase, inositol requiring enzyme-α (IRE1-α), protein kinase RNA-like endoplasmic reticulum kinase (PERK), α-tubulin, p-eIF2α, eIF2α, and GAPDH (Cell Signaling Technology); antiphospho-IRE1α (Thermo Scientific); antiacetylated histone H3 (Lys 18; Millipore); and XBP-1 or anti–α-actin (Santa Cruz Biotechnology) Abs.
RT-PCR
Total RNA from MM cell lines was purified with the RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions. Reverse transcription was carried out using Transcriptor Reverse Transcriptase (Life Technologies) with oligo-dT primer. cDNAs were then amplified using GoTaq Green Master Mix (Promega) with a pair of gene-specific primers. Primer sequences for the analyzed genes were as follows: XBP1 F:5′-CCTTGTAGTTGAGAACCAGG-3′, XBP1 R:5′-GGGGCTTGGTATATATGTGG-3′, GAPDH F:5′-TCCTCCTGTTCGACAGTCAGCCG-3′, and GAPDH R:5′-ACAGTTTCCCGGAGGGGCCAT-3.
MM xenograft mouse model (plasmacytoma model)
To evaluate the in vivo anti-MM activity of ACY-1215, male SCID mice were inoculated subcutaneously with 5 × 106 MM.1S cells in 100 μL of serum-free RPMI 1640 medium. When tumors were measurable, mice were treated IP with ACY-1215 50 mg/kg dissolved in 10% DMSO in 5% dextrose in water consecutively for 5 days a week for 3 weeks; bortezomib 0.5 mg/kg dissolved in 0.9% saline solution biweekly (IV) for 3 consecutive weeks; or combination with the same dosing regimen used for the individual agents. The control group received the carrier alone at the same schedule as the combination group. Tumor size was measured every other day in 2 dimensions using calipers, and tumor volume was calculated with the formula: V = 0.5(a × b2) where a is the long diameter of the tumor and b is the short diameter of the tumor. Mice were killed when the tumor reached 2 cm3 or was ulcerated. Survival and tumor growth were evaluated from the first day of treatment until death.
Disseminated MM model
To induce disseminated MM, female SCID-beige mice were inoculated intravenously with 5 × 106 MM.1S-LucNeo cells in 250 μL of PBS 18 days before treatment. On day 1, all animals were imaged to determine their tumor load before treatment. Mice were then separated into 4 treatment groups and received vehicle (n = 12), ACY-1215 75 mg/kg (n = 14) IP daily for 5 consecutive doses (days 1-5), bortezomib 1.5 mg/kg (n = 12) IP once weekly (day 3), or ACY-1215 75 mg/kg (n = 14) IP daily for 5 consecutive doses (days 1-5) and bortezomib 1.5 mg/kg (n = 12) IP administration once weekly (day 3; n = 15) for 2 consecutive weeks. Bioluminescence imaging was performed weekly to follow disease progression, and body weight changes were monitored daily.
PK/PD studies in plasmacytoma model
Male SCID mice were inoculated subcutaneously with 5 × 106 MM.1S cells in 100 μL of serum-free RPMI 1640 medium. When tumors reached 150-200 mm3, mice were treated intraperitoneally with vehicle or ACY-1215 50 mg/kg for 3 days and/or bortezomib 0.5 mg/kg IV once on day 3. Mice were killed 1, 4, and 24 hours after treatment; blood and tumor tissues were collected for immunohistochemistry (IHC), Western blotting (WB) analysis, and flow cytometry.
To assess the PK profile of ACY-1215, a minimum of 100 μL of blood plasma was collected from each animal. ACY-1215 was extracted from plasma by protein precipitation using 50:50 (vol/vol) acetonitrile: methanol. ACY-1215 was analyzed using a HPLC/MS/MS method using electrospray ionization in positive mode with multiple reaction transition monitoring. The lower and upper limit of quantification for ACY-1215 was 1-1000 ng/mL. For PD analysis, blood was collected from each animal as described in the preceding paragraph. Subcutaneous tumors (150-300 mm3) were harvested and analyzed by IHC, WB analysis, and flow cytometry.
Immunofluorescence assay
MM.1S cells were cultured on tissue culture–treated glass slides with or without 1μM ACY-1215 and/or 2.5nM bortezomib. After 12 hours, cells were fixed and permeabilized as described previously.30 After blocking, cells were stained with antiubiquitin Ab (Santa Cruz Biotechnology) 1:250 for 1 hour at room temperature. Cells were washed and incubated with Alexa Fluor 488 goat anti–mouse Ab (Invitrogen) for 1 hour. After subsequent washes, Hoechst 33342 (Invitrogen) was added for 10 minutes. The slides were mounted with Prolong Gold Antifade reagent (Invitrogen), and images were taken using a Nikon Ti-E microscope with 60× Plan-Apo VC/NA 1.4 oil lens equipped with an Andor Clara camera. Image acquisition, filter wheel and shutter were controlled by Andor iQ software. Images were merged with ImageJ 1.43u software. Images were taken at confocal and light microscopy core at Dana-Farber Cancer Institute.
IHC
IHC was performed using 5-mm-thick formalin-fixed paraffin embedded tissue sections. Slides were soaked in xylene, passed through graded alcohol, and placed in distilled water. Slides were pretreated with citrate buffer (Zymed) in a steam pressure cooker (Decloaking Chamber CD2008US; Biocare Biomedical) at the manufacturer's recommended settings. The slides were blocked for endogenous peroxidase activity with peroxidase block (Dako) as described previously.30
The mouse antiacetylated tubulin mAb (clone 6-11b-1; Sigma-Aldrich) or rabbit antiacetylated histone H3 (lysine 18) polyclonal Ab (Millipore) or rabbit antiacetylated-lysine polyclonal Ab (Cell Signaling Technology) was applied in Dako diluent at a 1:2500 dilution (for antiacetyl-tubulin), a 1:50 000 dilution (for antiacetyl-HH3), a 1:1000 dilution (for acetylated-lysine), or a 1:5000 dilution (for antiubiquitin) for 1 hour. After washing, the Ab was detected using the species-appropriate Envision kit (Dako) and diaminobenzidine, and then counterstained with Harris hematoxylin. Images were taken using Nikon Labophot-2 microscope with 10×/0.25 lens equipped with SPOT-insight QE camera.
For acetylated α-tubulin and acetylated histone H3 levels in MM patients, MM cells were purified by positive CD138 MicroBead selection, cultured with RPMI medium or 2μM ACY-1215 for 4 hours, washed, and fixed with 1% PFA for 1 hour at room temperature. After washing, cells were subjected to cytospin and stained with a combination of anti-CD138, antiacetylated α-tubulin, and antiacetylated histone H3 Ab. Images were photographed at a final magnification of 1000× using Olympus BX41 microscope, with 100×/1.30 oil lens, and Q-Color5 digital camera (Olympus Inc). Images were acquired using Adobe Photoshop CS4 software (Adobe Inc).
Statistical analysis
All in vitro experiments were performed in triplicate and repeated at least 3 times and a representative experiment was selected for the figures. The statistical significance of differences was determined using the Student t test with a minimal level of significance of P < .05. In vivo statistical tests were performed with 4 groups of 7 mice or more each using the 2-tailed Student t test. Overall survival (OS) was measured using the Kaplan-Meier method, and results are presented as the median OS with 95% confidence intervals. All statistical analyses were determined using Prism Version 5.0 software (GraphPad). Combination index values were calculated using the CompuSyn.exe Version 1.0.2004 program.
Results
ACY-1215 selectively inhibits HDAC6
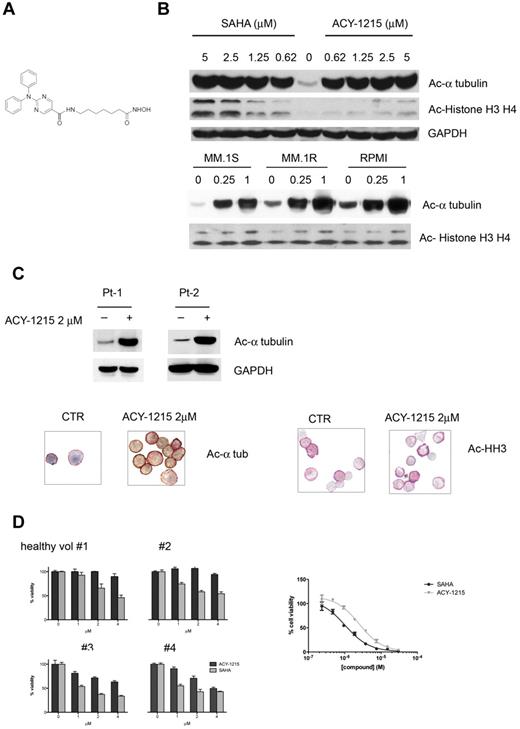
ACY-1215, a hydroxamic acid derivative (Figure 1A), demonstrated potent and selective inhibitory activity against HDAC6, with an enzymatic IC50 value of 5nM. ACY-1215 is 12-, 10-, and 11-fold less active against HDAC1, HDAC2, and HDAC3 (class I HDACs), respectively (Table 1). ACY-1215 has minimal activity (IC50 > 1μM) against HDAC4, HDAC5, HDAC7, HDAC9, HDAC11, Sirtuin1, and Sirtuin2, and has slight activity against HDAC8 (IC50 = 0.1μM).
ACY-1215 selectively inhibits HDAC6. (A) Chemical structure of ACY-1215. (B) MM.1S cells were cultured with control medium or ACY-1215 (0-5μM) or SAHA (0-5μM) for 6 hours. MM.1S, MM.1R, and RPMI8226 cells were cultured with control medium or ACY-1215 (0.25 and 1μM) for 18 hours. (C) CD138+ patient cells were treated with control medium or ACY-1215 (2μM) for 4 hours. Whole-cell lysates were subjected to Western blotting using the indicated Abs. Increased acetylated α-tubulin was observed. CD138+ patient cells were fixed and double-stained for anti–human CD138+ and acetylated α-tubulin (left panel) and for anti–human CD138+ and acetyl-histone H3 (right panel). A significant increase in acetylation of α-tubulin was observed, whereas no significant difference was observed in the acetyl-histone H3 in the treated sample compared with control. (D) PBMCs from 4 healthy donors were stimulated with PHA and cultured with increasing doses (0-4μM) of ACY-1215 and SAHA for 48 hours. Cell growth was assessed by MTT assay (left panel). CD4+ T cells purified from human blood were stimulated by CD3/CD28 Dynabeads for 7 days in the presence of compounds. Cell viability was assessed using alamarBlue (right panel)
ACY-1215 selectively inhibits HDAC6. (A) Chemical structure of ACY-1215. (B) MM.1S cells were cultured with control medium or ACY-1215 (0-5μM) or SAHA (0-5μM) for 6 hours. MM.1S, MM.1R, and RPMI8226 cells were cultured with control medium or ACY-1215 (0.25 and 1μM) for 18 hours. (C) CD138+ patient cells were treated with control medium or ACY-1215 (2μM) for 4 hours. Whole-cell lysates were subjected to Western blotting using the indicated Abs. Increased acetylated α-tubulin was observed. CD138+ patient cells were fixed and double-stained for anti–human CD138+ and acetylated α-tubulin (left panel) and for anti–human CD138+ and acetyl-histone H3 (right panel). A significant increase in acetylation of α-tubulin was observed, whereas no significant difference was observed in the acetyl-histone H3 in the treated sample compared with control. (D) PBMCs from 4 healthy donors were stimulated with PHA and cultured with increasing doses (0-4μM) of ACY-1215 and SAHA for 48 hours. Cell growth was assessed by MTT assay (left panel). CD4+ T cells purified from human blood were stimulated by CD3/CD28 Dynabeads for 7 days in the presence of compounds. Cell viability was assessed using alamarBlue (right panel)
Inhibition of HDAC enzymes
| Enzyme . | IC50, nM . | Potency (fold) vs HDAC6 . |
|---|---|---|
| HDAC1 | 58 | 12 |
| HDAC2 | 48 | 10 |
| HDAC3 | 51 | 11 |
| HDAC4 | 7000 | 1500 |
| HDAC5 | 5000 | 1100 |
| HDAC6 | 4.7 | |
| HDAC7 | 1400 | 300 |
| HDAC8 | 100 | 21 |
| HDAC9 | > 10 000 | > 2100 |
| HDAC11 | > 10 000 | > 2100 |
| Sirtuin 1 | > 10 000 | > 2100 |
| Sirtuin 2 | > 10 000 | > 2100 |
| Enzyme . | IC50, nM . | Potency (fold) vs HDAC6 . |
|---|---|---|
| HDAC1 | 58 | 12 |
| HDAC2 | 48 | 10 |
| HDAC3 | 51 | 11 |
| HDAC4 | 7000 | 1500 |
| HDAC5 | 5000 | 1100 |
| HDAC6 | 4.7 | |
| HDAC7 | 1400 | 300 |
| HDAC8 | 100 | 21 |
| HDAC9 | > 10 000 | > 2100 |
| HDAC11 | > 10 000 | > 2100 |
| Sirtuin 1 | > 10 000 | > 2100 |
| Sirtuin 2 | > 10 000 | > 2100 |
To confirm the specific inhibitory effect of ACY-1215 on HDAC6 activity, we first evaluated its effect on the acetylation of α-tubulin. MM.1S cells were cultured with increasing doses of ACY-1215 for 6 hours. A dose-dependent increased acetylated α-tubulin was observed at low doses (0.62μM) of ACY-1215 without affecting acetylation of histones, confirming its more selective inhibitory effect on HDAC6 activity (Figure 1B top panel) compared with SAHA. Similar acetylation selectivity for α-tubulin was observed in MM.1R and RPMI MM cell lines (Figure 1B bottom panel). This specific inhibitory effect of ACY-1215 on HDAC6 activity was next evaluated in primary MM cells. CD138+ patient MM cells were treated with or without 2μM ACY-1215 for 4 hours. WB analysis showed a significant increase of acetylated α-tubulin in treated compared with untreated cells (Figure 1C). To further evaluate the inhibitory effect of ACY-1215 on HDAC6 activity, CD138+ MM patient cells were fixed and double-stained with anti–human CD138 and with antiacetylated α-tubulin (Figure 1C left panel) or antiacetyl-histone H3 (Figure 1C right panel). We observed a significant increase of acetylated α-tubulin in treated compared with control cells, without any significant increase in acetyl-histone H3, confirming the selective inhibitory effect of ACY-1215 on HDAC6 activity.
We postulated that selectively targeting HDAC6 with ACY-1215 may result in less cytotoxicity to normal PBMCs than pan-HDAC inhibitors. To test this hypothesis, PBMCs from healthy donors were stimulated with PHA and cultured for 48 hours with increasing doses of either ACY-1215 or SAHA. ACY-1215 induced less cytotoxicity in PHA-stimulated PBMCs from 4 healthy donors compared with the pan-HDAC inhibitor SAHA (Figure 1D left panel). ACY-1215 also induced less cytotoxicity in unstimulated PBMCs (15% of toxicity for 4μM ACY-1215 vs 30% for 4μM SAHA; data not shown). The hypothesis was further confirmed on purified CD4+ T cells from human blood. The IC50 values for SAHA and ACY-1215 for T-cell toxicity were 1 and 2.5μM, respectively (Figure 1D right panel).
ACY-1215 induces dose-dependent cytotoxicity in MM cells alone or in coculture with cytokines or BMSCs
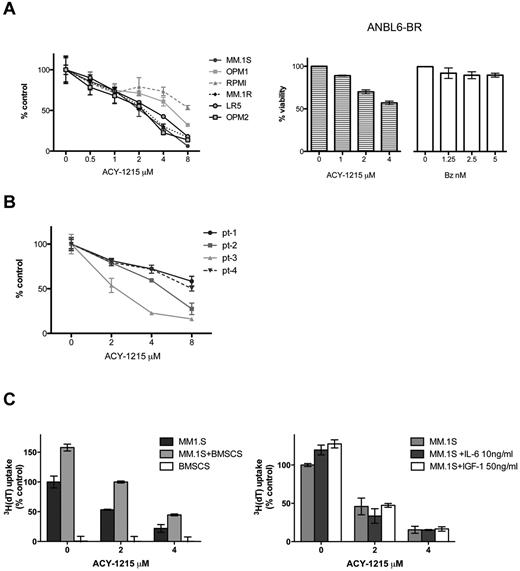
The effect of ACY-1215 on MM cell viability was next determined with MTT assays using MM cell lines. Exposure of MM cell lines for 48 hours resulted in dose-dependent decreased viability, with IC50 values ranging from 2-8μM (Figure 2A). ACY-1215 demonstrated significant activity in the MM bortezomib-resistant cell line (ANBL-6.BR), demonstrating the ability of ACY-1215 to overcome bortezomib resistance (Figure 2A right panel). Significant cytotoxicity was noted in the cells of 2 of 4 MM patients after 48 hours of treatment (Figure 2B). Because the BM microenvironment enhances growth and survival of MM cells,31,32 we next assessed the effect of ACY-1215 on MM.1S cells in the presence or absence of BMSCs, IL-6, or IGF-1. ACY-1215 decreased DNA synthesis of MM cells adherent to BMSCs at 48 hours in a dose-dependent manner and also inhibited growth induced by exogenous IL-6 and IGF-1 at 48 hours (Figure 2C). Therefore, ACY-1215 overcomes tumor cell growth and survival conferred by BMSCs and cytokines in the BM milieu.
ACY-1215 induces dose-dependent cytotoxicity in MM cells. (A) ACY-1215 decreases MM-cell viability in a dose-dependent manner. Cells were treated with increasing doses of ACY-1215 (0-8μM) for 48 hours, and cell viability was measured by MTT assay (left panel). ANBL-6.BR cells were treated with increasing doses of ACY-1215 (0.4μM) and bortezomib (0-5nM) to show their bortezomib resistance for 48 hours; cell viability was measured by MTT assay (right panel). (B) CD138+ patient MM cells (patients 1-4) were similarly tested in cytotoxicity assays (MTT) at 48 hours. (C) MM.1S cells were cultured for 48 hours with ACY-1215 (0-4μM) in the presence or absence of BMSCs (left) and in the presence or absence of IL-6 (10 ng/mL) and IGF-1 (50 ng/mL). 3H-thymidine incorporation was measured during the last 8 hours of incubation to measure DNA synthesis.
ACY-1215 induces dose-dependent cytotoxicity in MM cells. (A) ACY-1215 decreases MM-cell viability in a dose-dependent manner. Cells were treated with increasing doses of ACY-1215 (0-8μM) for 48 hours, and cell viability was measured by MTT assay (left panel). ANBL-6.BR cells were treated with increasing doses of ACY-1215 (0.4μM) and bortezomib (0-5nM) to show their bortezomib resistance for 48 hours; cell viability was measured by MTT assay (right panel). (B) CD138+ patient MM cells (patients 1-4) were similarly tested in cytotoxicity assays (MTT) at 48 hours. (C) MM.1S cells were cultured for 48 hours with ACY-1215 (0-4μM) in the presence or absence of BMSCs (left) and in the presence or absence of IL-6 (10 ng/mL) and IGF-1 (50 ng/mL). 3H-thymidine incorporation was measured during the last 8 hours of incubation to measure DNA synthesis.
ACY-1215 in combination with bortezomib induces synergistic anti-MM activity
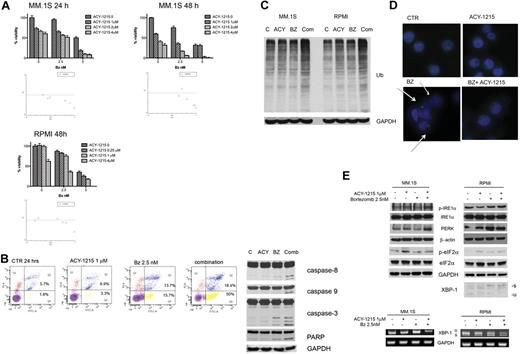
We next evaluated the activity of ACY-1215 in combination with bortezomib. Increasing doses of ACY-1215 (0-4μM) were added to increasing doses of bortezomib (0-5nM) for 24 and 48 hours in MM.1S cells and for 48 hours in RPMI8226 cells, and viability was then assayed by MTT (Figure 3A). A significant decrease in viability was observed after treatment with combined compared with single-agent therapy. Synergism was evaluated by applying the Chou-Talalay method.28 The combination of ACY-1215 and bortezomib showed synergistic anti-MM activity with a combination index < 1.0 (Figure 3A and table in supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). We next examined whether the combination of low-dose ACY-1215 plus bortezomib induced MM cell death, even in the presence of the BM microenvironment and cytokines. Combined therapy inhibited the uptake of tritiated thymidine in MM.1S cells cultured in the presence of BMSCs and cytokines (supplemental Figure 2). To characterize the mechanism of action of synergistic cytotoxicity induced by the combination treatment, we examined activation of apoptotic pathways by annexin V/PI staining. The analysis showed a significant increase (68.4%) of cells in early and late apoptosis after 24 hours treatment with combined therapy compared with either bortezomib (29.4%) or ACY-1215 (10.2%) alone (Figure 3B left panel). Apoptosis was confirmed by increased caspase-3, caspase-8, caspase-9, and poly(ADP-ribose) polymerase cleavage after 16 hours of treatment (Figure 3B right panel). Moreover, the combination of ACY-1215 plus bortezomib increased the accumulation of polyubiquitinated proteins compared with either agent alone (Figure 3C). To investigate the effect of ACY-1215 on aggresome formation, we treated MM.1S cells with 1μM ACY-1215 and/or 2.5nM bortezomib for 12 hours, and then cells were stained with immunofluorescent antiubiquitin Ab (Figure 3D). Bortezomib-treated cells showed perinuclear structures consistent with aggresome formation,8 which was disrupted when bortezomib and ACY-1215 were used in combination. This result indicates that inhibiting both the proteasome and aggresome pathways induces significant accumulation of polyubiquinated proteins in MM cells, as shown previously,5,8 and supports the potent synergistic anti-MM activity of ACY-1215 with bortezomib, which is consistent with our rationale for combined therapy.
ACY-1215 in combination with bortezomib induces synergistic anti-MM activity. (A) MM.1S and RPMI8226 cells were cultured with bortezomib in the presence or absence of ACY-1215 (0-4μM) for 24 and 48 hours. Cytotoxicity was assayed by MTT. The isobologram analysis confirms the synergistic effect. (B) MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 24 hours, followed by annexin/PI staining and flow cytometry analysis (left). MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 16 hours. Whole-cell lysates were immunoblotted with the indicated Abs. (C) MM.1S cells were treated with ACY-1215 (3μM), bortezomib (2.5nM), or combined therapy for 24 hours. Whole-cell lysates were immunoblotted with the indicated Abs. (D) MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 12 hours. Cells were fixed and stained with 4′,6-diamidino-2-phenylindole (blue) and antiubiquitin Ab. The arrow indicates the ubiquitin-conjugated proteins. (E) MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 4 hours. Whole-cell lysates were immunoblotted with the indicated Abs. XBP-1 splicing was determined by WB and RT-PCR.
ACY-1215 in combination with bortezomib induces synergistic anti-MM activity. (A) MM.1S and RPMI8226 cells were cultured with bortezomib in the presence or absence of ACY-1215 (0-4μM) for 24 and 48 hours. Cytotoxicity was assayed by MTT. The isobologram analysis confirms the synergistic effect. (B) MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 24 hours, followed by annexin/PI staining and flow cytometry analysis (left). MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 16 hours. Whole-cell lysates were immunoblotted with the indicated Abs. (C) MM.1S cells were treated with ACY-1215 (3μM), bortezomib (2.5nM), or combined therapy for 24 hours. Whole-cell lysates were immunoblotted with the indicated Abs. (D) MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 12 hours. Cells were fixed and stained with 4′,6-diamidino-2-phenylindole (blue) and antiubiquitin Ab. The arrow indicates the ubiquitin-conjugated proteins. (E) MM.1S cells were treated with ACY-1215 (1μM), bortezomib (2.5nM), or combined therapy for 4 hours. Whole-cell lysates were immunoblotted with the indicated Abs. XBP-1 splicing was determined by WB and RT-PCR.
It has been shown previously that bortezomib-induced apoptosis is associated with increased endoplasmic reticulum (ER) stress, activating the terminal unfolded protein response (UPR).33,34 In addition, HDAC inhibitors induce UPR and ER stress by abrogating formation of aggresomes, which normally serve to sequester and protect against misfolded polyubiquitinated proteins.5,35 In this context, we evaluated the effect of combined bortezomib and ACY-1215 therapy on IRE1, PERK, and XBP-1. MM.1S and RPMI cells cultured with ACY-1215 and/or bortezomib for 4 hours increased the expression of p-eIF2α, p-IRE1, and PERK, which is consistent with activation of ER stress. Moreover, in RPMI cells that clearly express the XBP-1 spliced form compared with MM.1S cells, 4 hours of combined therapy induced a modest activation of XBP-1, as evidenced by increased XBP-1 spliced-form mRNA and XBP-1s protein (Figure 3E). Therefore, our data suggest that combined therapy induces ER stress and UPR, which in turn trigger apoptosis.
ACY-1215 in combination with bortezomib induces anti-MM activity in vivo in a plasmacytoma model
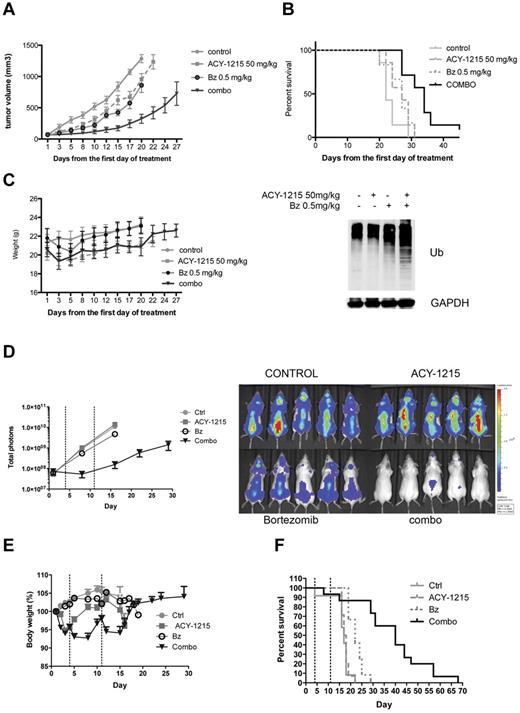
We next examined the in vivo efficacy of ACY-1215 in combination with bortezomib using a human MM xenograft mouse model.30 Mice treated with ACY-1215, bortezomib, or ACY-1215 plus bortezomib showed a significant delay in tumor growth (P = .01, P = .006, and P < .0001, respectively; Figure 4A). The delay in tumor growth was considerably more pronounced in the combination group compared with single-agent–treated groups (P = .0002 in ACY-1215 vs the combination and P = .0018 in bortezomib vs the combination). A significant prolongation in median OS was observed in the group treated with ACY-1215 plus bortezomib (Figure 4B; 22 days in the control vs 34 days in the treated group, P < .0011). No significant prolongation in median OS in the ACY-1215–treated group was observed (Figure 4B; 22 days in control vs 27 days in the treated group, P = .08) or in the bortezomib-treated group (Figure 4B; 22 days in control vs 27 days in the treated group, P = .14). The OS was significantly prolonged in the group treated with the combination compared with single agent alone (P = .02 in ACY-1215 vs combination and P = .01 in bortezomib vs the combination). WB analysis of tumors harvested from mice after 3 days of treatment showed a significant accumulation of polyubiquitinated proteins in the group treated with the combination of ACY-1215 plus bortezomib compared with either agent alone (Figure 4B). IHC analysis confirmed a significant increase in ubiquitin staining in the group treated with the combination compared with the single agents (supplemental Figure 3). Treatment with ACY-1215 plus bortezomib was well tolerated and did not significantly affect the body weight of the animals (Figure 4C). These results suggest that synergistic activity observed at the cellular level with ACY-1215 in combination with bortezomib (Figure 3A) translates to in vivo efficacy in the plasmacytoma model of MM. Furthermore, increased ER stress, as demonstrated by an increase in polyubiquinated proteins, was also confirmed in vivo.
ACY-1215 in combination with bortezomib induces significant anti-MM activity in vivo. (A) CB17 SCID mice were treated with saline (n = 7), ACY-1215 (50 mg/kg n = 7), bortezomib (0.5 mg/kg, n = 6), or the combination of ACY-1215 plus bortezomib (n = 7) for 3 weeks. Tumor growth was significantly inhibited in the combination-treated group compared with controls (P < .0001). (B) Using Kaplan-Meier and log-rank analysis, the median OS of animals treated with combination therapy was significantly prolonged (22 days in the control group vs 34 days in the treated group, P < .0011). WB analysis of tumors taken from mice after 3 days of treatment showed a significant accumulation of polyubiquitinated proteins in the group treated with the combination of ACY-1215 plus bortezomib compared with either agent alone (bottom panel). (C) Treatment with ACY-1215 or ACY-1215 plus bortezomib did not significantly affect the body weight of the animals. (D) SCID-beige mice were inoculated intravenously with MM.1S-LucNeo cells and then treated with saline (n = 10), ACY-1215 (n = 10), bortezomib (n = 10), or the combination of ACY-1215 plus bortezomib (n = 10) for 2 weeks. Combined treatment with ACY-1215 and bortezomib induced significant suppression of tumor growth, as demonstrated by bioluminescence imaging (log scale). (E) Treatment with ACY-1215 and ACY-1215 plus bortezomib induces between 4% and 12% of body weight loss. (F) Combined treatment with ACY-1215 and bortezomib significantly prolonged survival (17 days in the control group vs 40 days in the combination-treated group, P < .0001).
ACY-1215 in combination with bortezomib induces significant anti-MM activity in vivo. (A) CB17 SCID mice were treated with saline (n = 7), ACY-1215 (50 mg/kg n = 7), bortezomib (0.5 mg/kg, n = 6), or the combination of ACY-1215 plus bortezomib (n = 7) for 3 weeks. Tumor growth was significantly inhibited in the combination-treated group compared with controls (P < .0001). (B) Using Kaplan-Meier and log-rank analysis, the median OS of animals treated with combination therapy was significantly prolonged (22 days in the control group vs 34 days in the treated group, P < .0011). WB analysis of tumors taken from mice after 3 days of treatment showed a significant accumulation of polyubiquitinated proteins in the group treated with the combination of ACY-1215 plus bortezomib compared with either agent alone (bottom panel). (C) Treatment with ACY-1215 or ACY-1215 plus bortezomib did not significantly affect the body weight of the animals. (D) SCID-beige mice were inoculated intravenously with MM.1S-LucNeo cells and then treated with saline (n = 10), ACY-1215 (n = 10), bortezomib (n = 10), or the combination of ACY-1215 plus bortezomib (n = 10) for 2 weeks. Combined treatment with ACY-1215 and bortezomib induced significant suppression of tumor growth, as demonstrated by bioluminescence imaging (log scale). (E) Treatment with ACY-1215 and ACY-1215 plus bortezomib induces between 4% and 12% of body weight loss. (F) Combined treatment with ACY-1215 and bortezomib significantly prolonged survival (17 days in the control group vs 40 days in the combination-treated group, P < .0001).
ACY1215 in combination with bortezomib induces anti-MM activity in vivo in a disseminated MM model
The in vivo efficacy of combined therapy was further evaluated in a disseminated MM model.36 ACY-1215 alone showed no efficacy compared with vehicle in terms of tumor growth (Figure 4D, P = .22) and survival (Figure 4F, P = .55). Bortezomib alone showed moderate efficacy (approximately 60% reduction of tumor load by day 16), but only limited improvement in survival (Figure 4F; 17 days in the control group vs 22 days in the bortezomib-treated group, P < .0001). Combined treatment with ACY-1215 and bortezomib showed significant suppression of tumor growth (Figure 4D) and significantly prolonged OS compared with the control group (Figure 4F; 17 days in the control vs 40 days in the combination-treated group, P < .0001) and the bortezomib-treated group (Figure 4F; 22 days in the bortezomib group vs 40 days in the combination-treated group, P < .0001). Weight loss in the combination-treated group was between 4% and 12% compared with the same-day control group values during treatment, with complete recovery after the last injection (Figure 4E). As was observed in the plasmacytoma model, a significant therapeutic advantage was found by combining ACY-1215 with bortezomib compared with either agent alone.
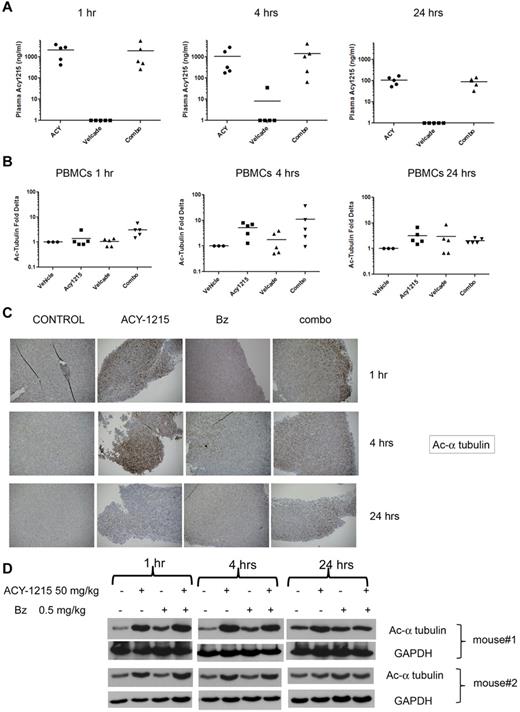
ACY-1215 PK/PD profile
In a separate study, we examined the in vivo PK and PD profile of ACY-1215 either as a single agent or in combination with bortezomib using the plasmacytoma mouse model30 described in “PK/PD studies in plasmacytoma model.” The mean plasma level of ACY-1215 at 1 hour after dose was 2133 and 1925 ng/mL in the groups treated with ACY-1215 (50 mg/kg) and with the combined therapy (ACY-1215 50 mg/kg plus bortezomib 0.5 mg/kg), respectively. The plasma concentration of ACY-1215 after 4 hours was 1079 and 1419 ng/mL in the group treated with ACY-1215 alone and in the group treated with combination therapy, respectively. After 24 hours, the mean plasma levels were 107 ng/mL in the single-agent group and 91 ng/mL in the combined-therapy group, showing a 10-fold decrease (Figure 5A) that reflects the elimination of ACY-1215. To characterize the PD activity of ACY-1215 in vivo, we used flow cytometry to evaluate the acetylation status of α-tubulin in PBMCs from each mouse. The maximum level of α-tubulin acetylation in PBMCs was observed 4 hours after administration of ACY-1215 alone or in combination (Figure 5B), which returned to near basal levels by 24 hours, reflecting the elimination of ACY-1215 in plasma. In addition, IHC analysis showed a significant increase in acetylation of α-tubulin staining 1 and 4 hours after administration of ACY-1215 and the combination (Figure 5C) on tumor tissue. These results were confirmed by WB analysis, which showed a significant increase in acetylated α-tubulin at 1 and 4 hours. IHC and WB analysis showed recovery to basal levels of acetylation of α-tubulin in the tumor tissue by 24 hours (Figure 5D). These data suggest that ACY-1215 rapidly distributes to the tumor tissue compartment in a manner consistent with plasma levels; clearance of ACY-1215 from tumor tissue parallels elimination of the drug from blood. There was no significant accumulation of the drug in tumor tissue. To confirm the selective inhibitory effect of ACY-1215 on HDAC6 activity in vivo, we evaluated the effect of treatment on the levels of acetylated lysine and acetylated histone H3 in tumor tissue. IHC analysis did not show significant increase in acetylated lysine (supplemental Figure 4A) or acetylated histone H3 (supplemental Figure 4B) staining at 1 and 4 hours after treatment with ACY-1215 and the combination. These results were further supported by WB analysis (supplemental Figure 3) and suggest that acetylated α-tubulin can be considered a specific target in MM cells for the activity of ACY-1215 alone and in combination with bortezomib.
ACY-1215 PK/PD profile. (A) CB17 SCID mice were treated with saline, ACY-1215, bortezomib, or the combination of ACY-1215 plus bortezomib for 3 days. ACY-1215 levels were detected in plasma collected from each animal at 1, 4, and 24 hours after the last dose. (B) The acetylation status of α-tubulin was assessed in mouse serum at 1, 4, and 24 hours after the last dose. The fold change in acetylation of α-tubulin relative to baseline was calculated. (C) Tumor was excised from each mouse at 1, 4, and 24 hours after the last dose. IHC analysis showed a significant increase in acetylation of α-tubulin staining at 1 and 4 hours after the last dose of ACY-1215 alone or in combination. (D) WB analysis confirmed a significant increase in acetylated α-tubulin at 1 and 4 hours after treatment with ACY-1215 alone or in combination.
ACY-1215 PK/PD profile. (A) CB17 SCID mice were treated with saline, ACY-1215, bortezomib, or the combination of ACY-1215 plus bortezomib for 3 days. ACY-1215 levels were detected in plasma collected from each animal at 1, 4, and 24 hours after the last dose. (B) The acetylation status of α-tubulin was assessed in mouse serum at 1, 4, and 24 hours after the last dose. The fold change in acetylation of α-tubulin relative to baseline was calculated. (C) Tumor was excised from each mouse at 1, 4, and 24 hours after the last dose. IHC analysis showed a significant increase in acetylation of α-tubulin staining at 1 and 4 hours after the last dose of ACY-1215 alone or in combination. (D) WB analysis confirmed a significant increase in acetylated α-tubulin at 1 and 4 hours after treatment with ACY-1215 alone or in combination.
Discussion
HDACs regulate the activity of tumor-suppressor genes and oncogenes that play crucial roles in tumorigenesis37 and therefore have been studied as therapeutic targets in solid tumors and hematologic malignancies, including MM.38,39 Small-molecule inhibitors of HDACs have been investigated in preclinical models in various cancer types.5-9,24,40 To date, both nonselective pan-HDAC inhibitors (such as SAHA) and specific class I HDAC inhibitors (such as romidepsin) have shown significant anti-MM activity in these models.38 Moreover, clinical studies of pan-HDAC inhibitors in combination with bortezomib have shown encouraging efficacy in MM.18,19 Based on previous studies, the synergistic activity of HDAC inhibitors plus bortezomib is due in part to the role played by HDAC6 in the aggresome pathway.5,8,24,41 Therefore, targeting both proteasomal and aggresomal protein degradation systems with proteasome inhibitor and HDAC inhibitors, respectively, induces massive accumulation of polyubiquitinated proteins, followed by activation of apoptotic cascades.5,41 These findings indicate that HDAC6 is a suitable target for selective inhibition, prompting the investigation of the preclinical activity of ACY-1215, a novel and selective inhibitor of HDAC6, either as a single agent or in combination with bortezomib in MM. Selective HDAC6 inhibition may reduce the toxicity related to the off-target effects of pan-HDAC inhibitors. Furthermore, it has been shown that mice with HDAC6 genetically knocked out are effectively normal, as opposed to genetic ablation of HDAC1, HDAC2, or HDAC3 (class I HDACs), which is lethal.42
The novel and selective HDAC6 inhibitor ACY-1215 was chosen from several lead candidates because of its properties that favor drug development. These criteria included at least 10-fold selectivity against HDAC6 compared with class I HDACs, minimal activity against other HDAC enzymes, and a lack of significant activity against an extensive panel of receptors, transporters, and enzymes, including kinases. Additional criteria included suitable oral bioavailability in rodents and nonrodents, cellular permeability, metabolic stability, and an appropriate in vitro safety profile with minimal drug-drug interaction, minimal potential for QTc prolongation (hERG channel), and no significant genotoxic signal in mammalian cells. Further, our data show that ACY-1215 was less toxic against PBMCs and T cells isolated from healthy volunteers compared with SAHA. We conclude that ACY-1215 was well tolerated in our animal studies.
To investigate the specific inhibitory effect of ACY-1215 on HDAC6 activity, we evaluated its effect on the acetylation of α-tubulin. Unlike all other HDACs, HDAC6 has substrate specificity for α-tubulin because of its α-tubulin deacetylase domain.43 ACY-1215 induces potent acetylation of α-tubulin at very low doses and triggers acetylation of lysine on histone H3 and histone H4 only at higher doses, confirming its specific inhibitory effect on HDAC6 activity. This specific inhibition was also observed in patient MM cells, in which ACY-1215 increased acetylated α-tubulin after 4 hours of treatment. However, after prolonged exposure or with significantly higher concentrations of ACY-1215, it is possible that the low level of class I HDACs (HDAC1, HDAC2, and HDAC3) inhibition by ACY-1215 may also contribute to MM cell cytotoxicity and to potent inhibition of HDAC6.
After evaluating the effect of ACY-1215 as a single agent in MM cell lines, we next focused our studies on the combination with bortezomib. A significant decrease in viability was observed with combined therapy compared with single agents alone, demonstrating potent synergism with the 2 agents, as anticipated from the suggested mechanism of action through inhibition of the proteasome and aggresome pathways.8,24 We tested the effect of ACY-1215 on aggresomes in bortezomib-treated MM cells and observed that ACY-1215 significantly disrupted aggresome formation with increased levels of polyubiquitinated protein. This is consistent with previous studies finding disrupted aggresome formation because of silencing of HDAC6.5,8 These data confirm the rationale of the combination therapy, which results in the inhibition of both proteasome and aggresome pathways with bortezomib and ACY-1215, respectively.
In addition to the above mechanism, bortezomib-induced apoptosis is associated with excessive ER stress, activating the terminal UPR.33 HDAC inhibitors also induce UPR and ER stress by abrogating formation of aggresomes, which normally serve to sequester and protect against misfolded polyubiquitinated proteins.34,35 Our data show that MM cell lines treated with ACY-1215 and/or bortezomib increase expression of p-IRE1 and PERK proteins and XBP-1 mRNA, which is consistent with activation of ER stress. However, eIF2α phosphorylation and XBP-1 splicing are not significantly up-regulated in the cells exposed to combined therapy versus single agents. These findings suggest that although ER stress and UPR may play a role in apoptosis induced by the combined therapy, they do not represent the principal mechanism involved in the observed synergy with ACY-1215 and bortezomib.
We also evaluated the in vivo anti-MM effect of combination therapy using 2 different xenograft models in SCID mice: a plasmacytoma model and a disseminated MM model. ACY-1215 in combination with bortezomib triggered more significant anti-MM activity than either agent alone in suppressing tumor growth and prolonging survival in both models, without significant adverse effects. As was observed in vitro, ACY-1215 in combination with bortezomib increased the level of polyubiquitinated proteins in tumor samples from the plasmacytoma model. These results clearly demonstrate the translation of the cellular findings with the 2 agents to established animal models of MM disease.
To optimize the design of future clinical trials and to validate a biomarker for this combination activity, we conducted PK and PD studies in our plasmacytoma model. Our data demonstrated peak plasma levels at 4 hours, which were not affected by the addition of bortezomib. To further characterize the activity of ACY-1215 against HDAC6 in vivo, we evaluated the acetylation of α-tubulin in mouse blood cells by flow cytometry. The maximum levels of blood-cell acetylation of α-tubulin were observed at 4 hours, providing an important biomarker for future clinical trials. The levels of acetylated α-tubulin were also detected in tumor tissue harvested from the mice in a similar time frame to peak blood levels, suggesting that ACY-1215 is readily absorbed by tumor tissue. Moreover, the drug did not accumulate in tumor tissue, as evidenced by the parallel decline of acetylated α-tubulin in blood cells and tumor tissue by 24 hours after dose.
Our in vitro data showed that ACY-1215 primarily induces acetylation of α-tubulin compared with histones H3 and H4 at pharmacologically active doses, confirming its HDAC6 selectivity versus class I HDACs. We further confirmed the selectivity in our in vivo models by investigating the effect of the drug combination on histone acetylation in tumor tissue. IHC and WB analysis did not show a significant increase in acetylated histone H3 (Lys 18) and lysine, while demonstrating a robust acetylation of α-tubulin, the primary marker of HDAC6 inhibition by ACY-1215 at the cellular level.
In conclusion, the results from our in vitro and in vivo studies show significant and synergistic anti-MM activity of ACY-1215, a novel and selective HDAC6 inhibitor, in combination with bortezomib and provide the rationale for advancement of this combination into clinical development. Moreover, the PD results provided here will help inform the design of correlative studies to accompany future clinical trials and establish whether acetylated α-tubulin can be used as predictive biomarker of HDAC6 inhibition and disease response.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
N.R. is a Leukemia & Lymphoma Society clinical scholar and recipient of the Claflin Distinguished Scholar Award. L.S. is a recipient of the Multiple Myeloma Research Foundation fellow award. K.C.A. is an American Cancer Society clinical research professor.
Authorship
Contribution: L.S. designed the research, performed the experiments, collected, analyzed, and interpreted the data, and wrote the manuscript; A.L.K. and J.-C.T. performed the experiments and collected and analyzed the data; S.C., H.E., D.C., T.S., M.C., D.T., M.Y., and M.J. performed the research and collected the data; S.J. provided compounds; S.J., T.H., J.B., J.v.D, W.C.O., R.M., and K.C.A contributed vital new reagents and analytical tools and helped with writing the manuscript; and N.R. designed research, provided the environment and support, and wrote the manuscript.
Conflict-of-interest disclosure: K.C.A. is an advisor for Celgene, Novartis, Millennium, Onyx, BMS, and Merck and is the founder of Acetylon Pharmaceuticals. T.H., A.L.K, and J.B. are consultants for Acetylon Pharmaceuticals. R.M. is cofounder and member of the scientific advisory board of Acetylon Pharmaceuticals. J.B. is a scientific founder, shareholder, and consultant for Acetylon Pharmaceuticals. M.J., D.T., M.Y., W.C.O., and S.J. are employees of Acetylon Pharmaceuticals. N.R. is on the advisory boards of Celgene, Novartis, Millennium, and Amgen. N.R. has received research funding from AstraZeneca and Acetylon Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Noopur Raje, MD, POB 216, MGH Cancer Center, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114; e-mail: nraje@partners.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal