Abstract
Circulating platelets contain high concentrations of TGF-β1 in their α-granules and release it on platelet adhesion/activation. We hypothesized that uncontrolled in vitro release of platelet TGF-β1 may confound measurement of plasma TGF-β1 in mice and that in vivo release and activation may contribute to cardiac pathology in response to constriction of the transverse aorta, which produces both high shear and cardiac pressure overload. Plasma TGF-β1 levels in blood collected from C57Bl/6 mice by the standard retro-bulbar technique were much higher than those obtained when prostaglandin E1 was added to inhibit release or when blood was collected percutaneously from the left ventricle under ultrasound guidance. Even with optimal blood drawing, plasma TGF-β1 was lower in mice rendered profoundly thrombocytopenic or mice with selectively low levels of platelet TGF-β1 because of megakaryocytespecific disruption of their TGF-β1 gene (Tgfb1flox). Tgfb1flox mice were also partially protected from developing cardiac hypertrophy, fibrosis, and systolic dysfunction in response to transverse aortic constriction. These studies demonstrate that plasma TGF-β1 levels can be assessed accurately, but it requires special precautions; that platelet TGF-β1 contributes to plasma levels of TGF-β1; and that platelet TGF-β1 contributes to the pathologic cardiac changes that occur in response to aortic constriction.
Introduction
TGF-β1 is a multifunctional cytokine that controls many biologic and pathologic functions, including cell proliferation and differentiation, the immune response, and tissue fibrosis.1-4 Elevated systemic levels of total TGF-β1 have been reported in a number of human disorders and in a number of murine models of human diseases, including coronary artery disease,5 aortic stenosis,6 Marfan syndrome,7 and malignancies.8 These data have been difficult to interpret, however, because the reported basal levels of TGF-β1 in control patients and mice have varied dramatically.5,9
Platelets contain 40 to 100 times as much TGF-β1 as other cells, and they rapidly release it on activation.10,11 Thus, in vitro release of TGF-β1 during blood collection and/or plasma preparation may confound the results of plasma TGF-β1 assays, such that they may not reflect the actual amount present in the systemic circulation. As a prelude to assessing the effects of platelet TGF-β1 on the cardiac response to pressure overload in a murine model involving constriction of the transverse aorta,12-14 we investigated different methods of blood collection and processing in mice. We monitored the in vitro release of platelet TGF-β1 in the samples by also measuring 2 other α-granule proteins that are normally present in plasma at a very low levels: platelet factor 4 (PF4) and thrombospondin-1 (TSP-1). To assess the platelet contribution to basal plasma levels of TGF-β1, we analyzed plasma TGF-β1 levels in control mice, in mice containing a conditional megakaryocyte-specific targeted deletion of TGF-β1, and in mice rendered acutely thrombocytopenic by injecting an anti-αIIbβ3 mAb. We hypothesized that platelet TGF-β1 may contribute to cardiac pathology in response to a surgical intervention that produces both a high shear state and pressure overload. To test this hypothesis, we studied the development of fibrosis and cardiac dysfunction in wild-type (WT) and the conditional gene targeted mice after inducing high shear and pressure overload by surgically constricting the transverse aorta.12-14
Methods
Materials
A chicken polyclonal IgY antibody to TGF-β1 was obtained from R&D Systems. The hamster anti–mouse αIIbβ3 mAb 1B5 was prepared as previously described.15 A goat polyclonal antibody to mouse PF4 was obtained from R&D Systems, and a mouse mAb to TSP-1 was obtained from Thermo Fisher Scientific. An isotype-matched hamster IgG was obtained from Jackson ImmunoResearch Laboratories, and a fluorescently labeled mAb to mouse GPIbβ was obtained from Emfret Analytics. A rabbit polyclonal antibody to mouse αIIb was obtained from Santa Cruz Biotechnology, and a rabbit polyclonal antibody to α-actin was obtained from Sigma-Aldrich. All other reagents and antibodies were from the same sources as previously reported.10
Mice
WT C57Bl/6 mice were obtained from The Jackson Laboratory. We generated C57Bl/6-inbred mice with TGF-β1-deficient platelets by interbreeding mice carrying a “floxed” TGF-β1 allele (Tgfb1flox; founder mice inbred for more than 6 generations to C57B1/6J were kindly provided by Dr Tom Deotschman, University of Arizona, Tucson, AZ)16 and subsequently crossed to C57Bl/6 transgenic mice expressing Cre recombinase under the control of the megakaryocyte-specific PF4 promoter (PF4CreTg+, kindly provided by Dr Radek Skoda, University Hospital Basel, Basel, Switzerland).17 All mice were housed in a controlled environment (23 ± 2°C; 12 hours light/dark cycles) and fed a standard diet (5001; Purina Mills). All experimental procedures were approved by The Rockefeller University and Cincinnati Children's Hospital Medical Center Animal Care and Use Committees. All mouse experiments were performed without the investigator knowing the genotype of the mice.
Hematologic data
Hematologic parameters were analyzed on EDTA or sodium citrate-anticoagulated blood within 30 minutes after blood collection using an automated dual-angle light scatter instrument (ADVIA120; Bayer Diagnostics) as described previously.18 When thrombocytopenia was detected in a sample, it was confirmed using flow cytometry with a fluorescently labeled mAb to mouse GPIbβ as previously described.18
Preparation of mouse platelet lysate, platelet releasate, serum, and plasma
Murine washed platelets were prepared as we previously described.19 Platelets (0.5 × 109) were stimulated with thrombin (0.125 U/mL) for 10 minutes at 37°C, and the releasates were collected as the supernatant after centrifuging the samples at 13 000g for 15 minutes at 4°C. Platelet pellets were lysed in lysis buffer (10mM Tris/HCl containing 1% Triton X-100) on ice for 30 minutes, and lysates were collected as the supernatant after centrifuging the sample at 13 000g for 20 minutes at 4°C as previously described.10
For the preparation of mouse serum, blood was collected in glass tubes without anticoagulant and incubated at 37°C for 4 hours; serum was collected as the supernatant after centrifuging the samples at 13 000g for 20 minutes at 4°C.
For the preparation of mouse plasma, blood was drawn by 2 different techniques: (1) retro-bulbar (RB) puncture from anesthetized mice, and (2) ultrasound-guided percutaneous left ventricle (LV) puncture. Blood obtained by the former technique was placed in a polypropylene tube containing 0.1 volume of 3.8% sodium citrate, pH 7.4. For the latter technique, animals were anesthetized with 2.5% isofluorane with oxygen and secured with medical tape to a heated stage that maintained their body temperature between 36°C to 37°C as measured by a rectal probe. ECG and heart rate were monitored continuously. A depilatory (Veet; Reckitt Benckiser) was used to remove the animals' hair on the thorax and upper abdomen. Using a 30-MHz ultrasound transducer (RMV707B; VisualSonics) and a long-axis view focused in B mode (Vevo 770; VisualSonics), the inflow tract of the LV was identified. A 27- to 30-gauge needle attached to a 1-mL syringe immobilized in an integrated rail system was then advanced into the middle of LV under continual ultrasound imaging of the tip of the needle. When the needle tip was properly positioned, free-flowing blood was collected into the syringe, which contained 0.1 volume of 3.8% sodium citrate. Preliminary studies of more than 200 control mice with this technique yielded a mortality rate of less than 1%.
Plasma was prepared from blood obtained by both techniques by centrifuging the sample either immediately or 2 hours after blood drawing. Centrifugation was performed at either 12 000g for 5 minutes (either at room temperature [RT] or 4°C) or at 3000g for 15 minutes (either at RT or 4°C) using a table-top microcentrifuge (Eppendorf). In some studies, PGE1 (1μM) was added to the blood collection tube before blood collection to prevent release of platelet granule contents during plasma preparation.
Induction of thrombocytopenia in mice
Mice were injected intraperitoneally with 0.1 to 1.0 mg/kg of the anti-αIIbβ3 mAb 1B5. Blood samples were obtained 24 hours, 48 hours, and 72 hours after injection by the LV puncture method. Whole blood platelet count was determined with an automated instrument (ADVIA120) and by flow cytometry using a fluorescently labeled mAb to mouse GPIbβ.18 Plasma samples were prepared by immediately centrifuging the samples at 12 000g for 5 minutes at RT. No platelet contamination was observed in the plasma prepared by this method.
Measurement of TGF-β1
Total TGF-β1 in platelets, serum, and plasma was measured after converting latent TGF-β1 to active TGF-β1 by acidification (20-minute incubation at RT with 0.5 volume of 1 N HCl for plasma and serum and 0.2 volume of 1 N HCl for platelet lysates and releasates, followed by neutralization by adding the same volume of 1.2 N NaOH in 0.5M HEPES) with a 2-antibody ELISA assay specific for the activated form of TGF-β1 (R&D Systems). Serum samples were diluted 1:200, plasma samples were diluted 1:10 to 1:20, and platelet samples (lysates and releasates) were diluted 1:100.
TGF-β1 activity was also measured as previously described10 by a functional bioassay, using a mink lung epithelial cell line stably expressing a luciferase reporter gene under the control of the plasminogen activator inhibitor-1 promoter (kindly provided by Dr Daniel Rifkin, NYU Medical School, NY). This assay previously was reported to have a lower limit of detection of 2 pg/mL TGF-β1,20 and we independently found it to have the same value.
Immunoblotting
Platelet releasates, platelet lysates, or serum or plasma samples were mixed with 2× SDS sample buffer, heated to 100°C for 3 minutes, and electrophoresed in 8% to 16% gradient Tris-glycine gels (Invitrogen). After protein transfer to PVDF membranes, selected proteins were immunoblotted using antibodies to mouse PF4 or TSP-1 and detected using secondary antibodies conjugated to HRP and the ECL chemiluminescence detection system (GE Healthcare). Band intensities were quantitated using image analysis software (National Institutes of Health–Scion Image).
FeCl3-induced thrombosis model
To test whether megakaryocyte/platelet-specific reduction of TGF-β1 expression affects platelet function in vivo, we used an FeCl3-induced carotid artery thrombosis model as previously described.10 Briefly, thrombosis was induced by applying a filter paper soaked with 20% FeCl3 to the exposed segment of the carotid artery for 3 minutes. The time to artery occlusion after exposure to FeCl3 was then monitored.
Platelet aggregation
In vitro platelet aggregation was evaluated over a 10-minute time period at 37°C in a photometric aggregometer (Kowa Optimed). Washed platelets from WT and Tgfb1flox mice were stirred, and aggregation was induced by either ADP (1μM) or thrombin (0.125 U/mL).
TAC model
Transverse aortic constriction (TAC) was induced by controlled constriction of the transverse aortic arch as described previously.12-14 Briefly, mice were anesthetized by isoflurane, and the aorta was exposed and constricted at the mid aortic arch level with a 7/0 silk ligature using a blunted 27-gauge (0.41-mm OD) needle as calibrator, producing an approximately 70% reduction in luminal diameter. Sham control mice underwent the same procedure but without the aortic constriction. Blood samples were obtained before surgery (Pre) and 1 and 4 weeks after surgery under ultrasound guidance from the left ventricle inflow tract using a 27-gauge needle and 1-mL syringe containing the anticoagulant. After 4 weeks, the animals were anesthetized and euthanized; the hearts were then excised, perfused with saline, weighed, and fixed in 4% paraformaldehyde for histology. Myocardial fibrosis was evaluated histologically by staining with hematoxylin and eosin and Masson trichrome. Grading of fibrosis on a scale of 0 to 4 was performed by an expert veterinary pathologist without knowledge of the treatment. Dual immunohistochemical staining with rabbit anti-vimentin and mouse anti-α smooth muscle actin (both from Abcam) was performed using a Polink-DS kit according to the manufacturer's protocol (Golden Bridge International).
Echocardiographic measurements
Because the depth of anesthesia and changes in body temperature have previously been reported to affect cardiac physiologic measurements,21 all measurements were performed under relatively mild anesthesia and at constant body temperature. Mice were anesthetized with inhaled isoflurane (1.5%-2.0%; Aerrane, Baxter) in 1 L/min 100% O2, secured on a heating stage, and maintained at a body temperature between 36°C and 37°C with the aid of a rectal temperature probe. Heart rate and ECG were continuously monitored (THM100, Indus Instruments). Using B-mode imaging (Vevo770; VisualSonics), the transducer (30-MHz RMV707B) was positioned to image longitudinal or cross sections of the heart. Subsequently, the regions of interest were focused and cine loops were recorded for analysis. Flow measurements were obtained using the pulse-wave Doppler mode after adjusting the transducer's position to keep the angle of correction less than 45°. All echocardiographic measurements were made 3 times and the average values are reported.
Systolic function indices.
The following parameters were measured at end-diastole (d) and end-systole (s) using M-mode images at the level of the papillary muscles in a left parasternal short axis view: interventricular septal wall thickness (IVS), LV internal dimensions (LVID), and posterior wall thickness (PW; IVSd, LVIDd, PWd, and IVSs, LVIDs, PWs). LV fractional shortening was calculated as previously described by Syed et al.22
Because adult mice have heart rates of approximately 500 to 650 beats per minute without anesthesia, it is difficult to separate systole from diastole when imaging at 100 frames per second by B-mode imaging. To overcome this limitation, we used ECG-gated kilohertz visualization, which gives 1000 frames per second.23 Using these images, we first measured: (1) endocardial and epicardial areas at end-systole and end-diastole; and (2) LV base to apex distance at end-systole and end-diastole (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Based on these measurements, the following parameters were calculated: (1) LV end-systole and end-diastole volumes, (2) fractional shortening (FS), (3) ejection fraction (EF), (4) stroke volume (SV), (5) average wall thickness at end-diastole, and (6) LV mass (supplemental Figure 1B). The contractile state of the myocardium, independent of heart rate and preload, was estimated by calculating the corrected velocity of circumferential fiber shortening (Vcfc).22,24
Diastolic function indices.
The transmitral flow profile was recorded in an apical 4-chamber view sound window using pulse-wave Doppler echocardiography to measure the following parameters: (1) E- and A-wave peak velocities, (2) E-wave deceleration and acceleration time, (3) isovolumetric relaxation and contraction time, (4) ejection time, and (5) velocity time integral. Using these parameters, the E/A ratio and the myocardial performance index were calculated as described previously.25,26
Vessel diameter and flow profiles.
The diameters of the ascending and transverse aorta and the innominate artery were measured at end-diastole and at end-systole using B-mode images. Flow velocity was measured using the pulse-wave-Doppler flow mode view.
Real-time PCR.
Total RNA was extracted from heart tissue 4 weeks after sham or TAC surgery using RNeasyFibrous Tissue Mini Kit (QIAGEN). cDNA was prepared from the RNA (High Capacity RNA-to-cDNA Kit; Applied Biosystems). Real-time PCR was performed with ready-made primer sets for mouse collagen 1, collagen 3, and Tgfb1 genes (Applied Biosystems) using an Applied Biosystems 7900-HT real time PCR system. The thermal cycler conditions were 50°C for 2 minutes, followed by 95°C for 10 minutes and 95°C for 15 seconds, and finally 60°C for 1 minute. A total of 40 cycles were run. Data were normalized to the endogenous control gene Gapdh and analyzed using SDS Version 2.4 and RQ Manager Version 1.2.1 software (Applied Biosystems).
Statistical analysis
All continuous data are reported as mean and 95% CI if they were normally distributed, and as median and interquartile range if they were not normally distributed, unless otherwise specified. Before applying parametric statistics, data were checked for the assumptions of normality. Differences in means between 2 independent groups were analyzed using a 2-sample Welch t test, whereas paired Student t test was used with paired samples. The Wilcoxon rank-sum test was used for data that were not normally distributed and the rank-sum test was used for paired samples. Heart weight/body weight ratios were analyzed using a 2-way ANOVA with the genotype, surgery type, and genotype-surgery interaction as fixed factors. Pearson product-moment correlation was used to correlate continuous values with underlying normality and sufficient sample size; otherwise, Spearman rank correlation ρ was used. For assessing the results of the longitudinal TAC study, a linear mixed model was used, with the surgery type, time point, and genotype as fixed effects and mouse as the random effect. AR1 was used as the variance-covariance structure. Group effects of the resulting model-fit were tested via an ANOVA; multiple comparisons were performed by analyzing the estimate contrasts in the model. A 2-tailed P value of < .05 was considered significant. Statistical analyses were performed using R Version 2.1.12; plots were created with ggplot2.27,28
Results
Ultrasound-guided LV puncture blood drawing minimizes in vitro release of TGF-β1 compared with RB blood collection
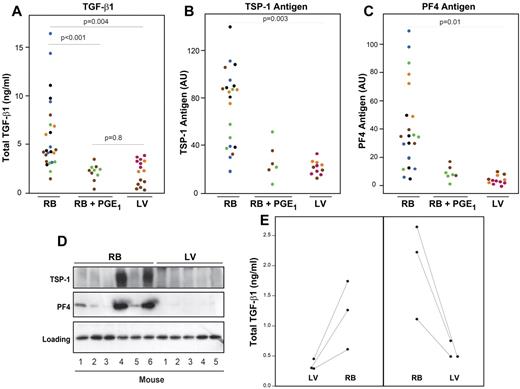
We investigated the effect of different methods of blood drawing and plasma preparation on TGF-β1 levels in mice (Figure 1). All blood samples were collected into syringes or tubes containing 0.1 volume of 3.8% sodium citrate and centrifuged within 2 to 5 minutes at 3000g for 15 minutes at RT. Total TGF-β1 plasma levels were 5.9 ± 3.7 ng/mL (n = 25) using the RB technique without PGE1 and approximately 70% lower (2.1 ± 0.8 ng/mL; P < .001; n = 10) when PGE1 was added to the tube in which the blood was collected (Figure 1A). Plasma levels using the LV method were similar to those using the RB method in the presence of PGE1 (2.2 ± 1.2 ng/mL; n = 15; P = .8).
The ultrasound-guided LV puncture method of blood drawing minimizes in vitro release of TGF-β1, PF4, and TSP-1 from platelet α-granules into plasma. (A) Blood samples were drawn into sodium citrate anticoagulant by either the RB capillary technique in the absence (RB; n = 25) or presence of 1μM PGE1 (RB + PGE1; n = 10), or by the ultrasound-guided LV method (n = 15). Plasma samples were prepared by centrifuging blood at 3000g for 15 minutes at RT. Total TGF-β1 was measured by ELISA. (B) TSP-1 and (C) PF4 antigen levels were estimated by measuring immunoreactive band intensities on immunoblots. Each dot represents an individual mouse sample, and each color represents data obtained from the same mouse. P values reflect RB values compared with LV values. (D) Representative immunoblots with antibodies to TSP-1 and PF4 of plasma prepared by the RB method from 6 mice and by the LV methods from 5 different mice. Bottom panel: A nonspecific band, indicating that similar amounts of protein were loaded in each lane. (E) Total TGF-β1 was measured in plasma prepared from blood collected first by the LV method and subsequently by the RB method (left panel) or from blood collected in the opposite sequence (right panel).
The ultrasound-guided LV puncture method of blood drawing minimizes in vitro release of TGF-β1, PF4, and TSP-1 from platelet α-granules into plasma. (A) Blood samples were drawn into sodium citrate anticoagulant by either the RB capillary technique in the absence (RB; n = 25) or presence of 1μM PGE1 (RB + PGE1; n = 10), or by the ultrasound-guided LV method (n = 15). Plasma samples were prepared by centrifuging blood at 3000g for 15 minutes at RT. Total TGF-β1 was measured by ELISA. (B) TSP-1 and (C) PF4 antigen levels were estimated by measuring immunoreactive band intensities on immunoblots. Each dot represents an individual mouse sample, and each color represents data obtained from the same mouse. P values reflect RB values compared with LV values. (D) Representative immunoblots with antibodies to TSP-1 and PF4 of plasma prepared by the RB method from 6 mice and by the LV methods from 5 different mice. Bottom panel: A nonspecific band, indicating that similar amounts of protein were loaded in each lane. (E) Total TGF-β1 was measured in plasma prepared from blood collected first by the LV method and subsequently by the RB method (left panel) or from blood collected in the opposite sequence (right panel).
To assess in vitro release of other α-granule contents, we also tested plasma levels of PF4 and TSP-1 antigens by immunoblotting. The RB method was associated with variable amounts of plasma PF4 and TSP-1, with some samples staining strongly and others weakly (Figure 1B-D). In sharp contrast, plasma levels of PF4 and TSP-1 were consistently lower in the samples with PGE1 and the samples obtained by the LV method.
We further compared different centrifugation methods and found lower levels of plasma TGF-β1 when blood drawn by the LV method was centrifuged immediately at 12 000g for 5 minutes at RT (0.56 ± 0.2 ng/mL; n = 6) than when centrifuged at 3000g for 15 minutes at RT (2.2 ± 1.2 ng/mL; n = 15; P < .001). We then assessed the effect of drawing blood sequentially by the RB and LV methods in 2 sets of mice (Figure 1E). In one group of mice, blood was initially obtained by the LV method and then obtained by the RB technique; in the other group of mice, blood was obtained in the opposite sequence. All samples were centrifuged at 12 000g for 5 minutes at RT. In both groups of mice, plasma prepared from blood drawn by the LV method had significantly lower levels of TGF-β1 (0.56 ± 0.2 ng/mL vs 1.6 ± 0.8 ng/mL, n = 6; P < .005).
Induction of profound thrombocytopenia in mice increases skin bleeding and decreases plasma levels of TGF-β1
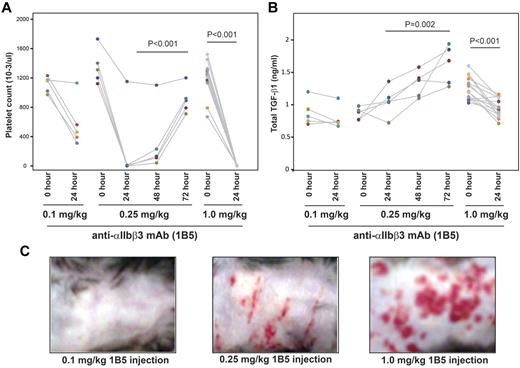
WT C57BL/6 mice were injected with different concentrations of anti-αIIbβ3 mAb 1B5 to induce thrombocytopenia. Injection of 0.1 mg/kg 1B5 led to an approximately 50% reduction in platelet counts 24 hours after injection (Figure 2A), which was not associated with clinical evidence of hemorrhage (Figure 2C left panel) or a change in hematocrit (data not shown). Increasing the dose of 1B5 to 0.25 mg/kg led to a more than 90% reduction in platelet count 24 hours after injection, after which platelet counts in 4 of 5 animals started to increase by 48 hours. This dose of 1B5 was associated with petechial bleeding in all animals (Figure 2C middle panel) and from the nose in 1 mouse. The hematocrits in these mice were decreased 48 hours after injection (data not shown). By 72 hours after injection, the platelet count in all mice returned to approximately 60% of the pretreatment value (Figure 2A). Increasing the 1B5 dose to 1.0 mg/kg led to nearly complete platelet depletion (> 99%) 24 hours after injection (Figure 2A). This treatment was associated with extensive, confluent ecchymoses in all animals (Figure 2C right panel), nosebleeds in 3 of 12 animals, and lethal hemothorax in all animals immediately after blood drawing under ultrasound guidance (data not show).
Induction of profound thrombocytopenia by mAb 1B5 injection reduces plasma TGF-β1 levels in mice. WT C57BL/6 mice were injected intraperitoneally with the indicated amount of hamster mAb 1B5 against mouse αIIbβ3. (A) Platelet counts at the indicated time points after injection of 1B5. (B) Total TGF-β1 levels in plasma samples at the indicated time points as measured by ELISA. Each dot represents an individual mouse, and the different colors indicate samples from different mice. (C) Bleeding over the thorax and abdomen 24 hours after the 1B5 injection.
Induction of profound thrombocytopenia by mAb 1B5 injection reduces plasma TGF-β1 levels in mice. WT C57BL/6 mice were injected intraperitoneally with the indicated amount of hamster mAb 1B5 against mouse αIIbβ3. (A) Platelet counts at the indicated time points after injection of 1B5. (B) Total TGF-β1 levels in plasma samples at the indicated time points as measured by ELISA. Each dot represents an individual mouse, and the different colors indicate samples from different mice. (C) Bleeding over the thorax and abdomen 24 hours after the 1B5 injection.
Plasma total TGF-β1 levels were not affected by the 0.1-mg/kg dose despite the approximately 50% decrease in platelet counts in 4 of 5 mice, suggesting that in vitro release of platelet TGF-β1 during blood drawing and/or sample preparation did not make a major contribution to the plasma levels. In contrast, plasma levels of total TGF-β1 exhibited an approximately 50% to 100% increase over 72 hours in the mice treated with 0.25 mg/kg, but an approximately 28% decrease in the first 24 hours in mice treated with the 1.0-mg/kg dose (Figure 2B).
Mice with megakaryocyte-specific targeted deletion of TGF-β1 are viable and do not show any apparent defect in platelet function in vitro or in vivo
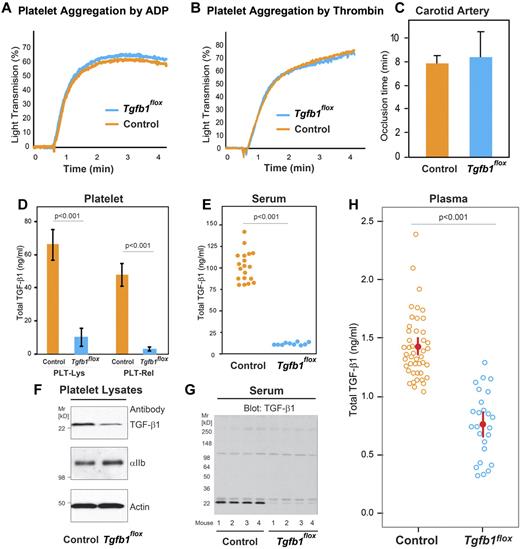
Mice with megakaryocyte-specific targeted deletion of the TGF-β1 gene (PF4CreTg+/Tgfb1flox/flox; designated Tgfb1flox for simplicity) were born at the expected Mendelian frequency, survived well into their adulthood, and did not exhibit any overt abnormalities. This is in sharp contrast to the early death, usually within 3 weeks of birth, of mice with nonspecific targeted deletion of the TGF-β1 gene.29,30 Hematologic values (including platelet counts and mean platelet volumes) in Tgfb1flox mice were similar to those observed in control mice (supplemental Table 1). Platelet aggregation responses induced by ADP (Figure 3A) or thrombin (Figure 3B) were also similar in Tgfb1flox and control mice. Exposure of arteries from both control (n = 4) and Tgfb1flox (n = 4) mice to 20% FeCl3 resulted in complete occlusion of the vessels in approximately 7 to 8 minutes (Figure 3C).
Megakaryocyte-specific targeted deletion of TGF-β1 reduces platelet, platelet releasate, serum, and plasma levels of total TGF-β1 but does not affect platelet function in vitro or in vivo. (A-B) Agonist-induced platelet aggregation was similar in control and Tgfb1flox mice. (A) Platelet-rich plasma from control and Tgfb1flox mice was stimulated with 1μM ADP, and light transmission was measured over time (1 of 3 similar experiments is shown). (B) Washed platelets from control and Tgfb1flox mice were stimulated with 0.125 U/mL thrombin, and the aggregation response was measured over time (1 of 3 similar experiments). (C) FeCl3-induced carotid artery thrombosis in vivo was similar in control and Tgfb1flox mice. Thrombosis was initiated by applying 20% FeCl3 to the carotid arteries of control and Tgfb1flox mice, and the time to complete cessation of blood flow was recorded as the occlusion time (n = 4). (D) Total TGF-β1 levels in platelet lysates (PLT-Lys) and platelet releasates (PLT-Rel) from control mice (n = 4) or from Tgfb1flox (n = 4) were measured by ELISA. (D) Serum total TGF-β1 levels in control (n = 19) and Tgfb1flox mice (n = 10) were measured by ELISA. (E) Platelet lysates from control and Tgfb1flox mice were immunoblotted with antibodies to TGF-β1 (top panel), αIIb (middle panel), and actin (bottom panel). (F) Serum samples from 4 control and 4 Tgfb1flox mice were immunoblotted with antibody to TGF-β1. (G) Plasma total TGF-β1 levels in control (n = 45) and Tgfb1flox (n = 25) mice were measured by ELISA.
Megakaryocyte-specific targeted deletion of TGF-β1 reduces platelet, platelet releasate, serum, and plasma levels of total TGF-β1 but does not affect platelet function in vitro or in vivo. (A-B) Agonist-induced platelet aggregation was similar in control and Tgfb1flox mice. (A) Platelet-rich plasma from control and Tgfb1flox mice was stimulated with 1μM ADP, and light transmission was measured over time (1 of 3 similar experiments is shown). (B) Washed platelets from control and Tgfb1flox mice were stimulated with 0.125 U/mL thrombin, and the aggregation response was measured over time (1 of 3 similar experiments). (C) FeCl3-induced carotid artery thrombosis in vivo was similar in control and Tgfb1flox mice. Thrombosis was initiated by applying 20% FeCl3 to the carotid arteries of control and Tgfb1flox mice, and the time to complete cessation of blood flow was recorded as the occlusion time (n = 4). (D) Total TGF-β1 levels in platelet lysates (PLT-Lys) and platelet releasates (PLT-Rel) from control mice (n = 4) or from Tgfb1flox (n = 4) were measured by ELISA. (D) Serum total TGF-β1 levels in control (n = 19) and Tgfb1flox mice (n = 10) were measured by ELISA. (E) Platelet lysates from control and Tgfb1flox mice were immunoblotted with antibodies to TGF-β1 (top panel), αIIb (middle panel), and actin (bottom panel). (F) Serum samples from 4 control and 4 Tgfb1flox mice were immunoblotted with antibody to TGF-β1. (G) Plasma total TGF-β1 levels in control (n = 45) and Tgfb1flox (n = 25) mice were measured by ELISA.
Megakaryocyte-specific gene targeting of TGF-β1 reduces platelet, platelet releasate, and serum levels of TGF-β1
Total TGF-β1 levels in platelet lysates prepared from blood obtained by the LV method were approximately 85% lower in Tgfb1flox mice (10.0 ± 5.0 ng of total TGF-β1/109 platelets) than in control mice (66.0 ± 10 ng/109 platelets; P < .001; n = 4). Similarly, total TGF-β1 levels in platelet releasates were approximately 94% lower in Tgfb1flox mice (3.0 ± 1.0 ng/109 platelets; n = 4) than control mice (48.0 ± 7.0 ng/109 platelets; n = 4; P < .001; Figure 3D). Serum TGF-β1 levels were approximately 87% lower in Tgfb1flox mice than in control mice (12.0 ± 2.0 ng/mL, n = 10; vs 94.0 ± 25.0 ng/mL, n = 19; P < .001; Figure 3E). TGF-β1 immunoblots of platelet lysates prepared from control and Tgfb1flox mice confirmed the results from the ELISA by revealing less intense staining in the Tgfb1flox sample (Figure 3F top panel). Immunoblot staining intensities for the platelet-specific protein αIIb and actin were similar in both control and Tgfb1flox mice (Figure 3F middle and bottom panels, respectively), indicating that the same amount of protein was loaded in each sample. Immunoblotting also revealed lower serum levels of TGF-β1 in Tgfb1flox mice than in control mice (Figure 3G).
Megakaryocyte-specific gene targeting of TGF-β1 reduces plasma levels of TGF-β1
Total TGF-β1 levels in plasma samples prepared by the LV method were approximately 45% lower in Tgfb1flox mice than in control mice (median, 0.76 ng/mL; interquartile range, 0.64-0.88 ng/mL, n = 25; vs median, 1.37 ng/mL; interquartile range, 1.23-1.56 ng/mL, n = 45; P < .001; Figure 3H).
In separate experiments, we compared the effect of centrifuging blood from C57BL/6 (WT) and Tgfb1flox mice immediately after collection at RT or at 4°C on plasma TGF-β1 levels. We found no significant difference in total TGF-β1 levels between these conditions: total TGF-β1 levels were 1.5 ± 0.4 ng/mL at RT versus 1.3 ± 0.3 ng/mL at 4°C in WT mice (n = 5; P = .4) and 0.9 ± 0.2 ng/mL at RT (n = 3) versus 0.9 ± 0.2 ng/mL at 4°C (n = 7) in Tgfb1flox mice (P = .9). We also tested the effect of adding PGE1 to samples from both WT and Tgfb1flox mice and found that the addition of PGE1 did not reduce TGF-β1 levels further when samples were prepared by centrifuging at 12 000g at 4°C: total TGF-β1 levels were 1.3 ± 0.3 ng/mL without and 1.2 ± 0.2 ng/mL with PGE1 in WT mice (n = 5; P = .9) and 0.9 ± 0.2 ng/mL without and 1.0 ± 0.3 ng/mL with PGE1 in Tgfb1flox mice (n = 7; P = .6).
Mice with megakaryocyte-specific gene targeting of TGF-β1 have larger major blood vessels than WT mice but have similar cardiac function
Tgfb1flox mice (n = 23) were slightly heavier (27 g, n = 25; vs 26 g, n = 27) than WT mice (P = .02) before surgery, but there was no difference in body weight at the end of the experiment. The diameters of the aortic arch and innominate artery were larger in Tgfb1flox mice compared with WT mice in both end-systole and end-diastole (1.6 vs 1.4 mm at end-systole and 1.3 vs 1.1 mm at end-diastole; P < .001 for WT vs Tgfb1flox mice for both end-systole and end-diastole), and this difference persisted after adjusting for body weight. Despite being slightly heavier, LV wall thickness at end-diastole and LV wall mass were lower in Tgfb1flox mice (n = 23) compared with WT control mice (n = 42; P < .001). WT and Tgfb1flox mice had similar cardiac function as judged by SV, EF, and fractional shortening (FS; supplemental Table 2).
Tgfb1flox mice are partially protected from developing TAC-induced cardiac hypertrophy, fibrosis, and systolic dysfunction
Cardiac pressure overload was induced by TAC in 23 C57Bl/6 and 13 Tgfb1flox mice using a ligature in combination with a blunted 27-gauge needle as calibrator. Two WT mice were excluded from analysis because they were found to have incomplete stenotic lesions (both ∼ 0.6 mm). Mice from both groups (n = 21 and n = 10, respectively) also underwent sham surgery and served as controls. Two Tgfb1flox mice undergoing sham surgery were excluded from analysis because they were found to have aortic stenosis on baseline echocardiographic analysis. In the animals included in the study, the stenosis reduced the aortic diameter from 1.31 mm (n = 10) to 0.26 mm (n = 11) in WT mice and from 1.43 mm (n = 9) to 0.29 mm (n = 11) in Tgfb1flox mice; the degree of stenosis remained unchanged throughout the 4 weeks of the experiment. There were no significant differences in the size of the innominate artery, the mean pressure gradient across the stenosis (P = .20), the mean velocity of blood flow across the stenosis (P = .19), or peak velocity across the stenosis (P = .75) 1 week after surgery (supplemental Table 3).
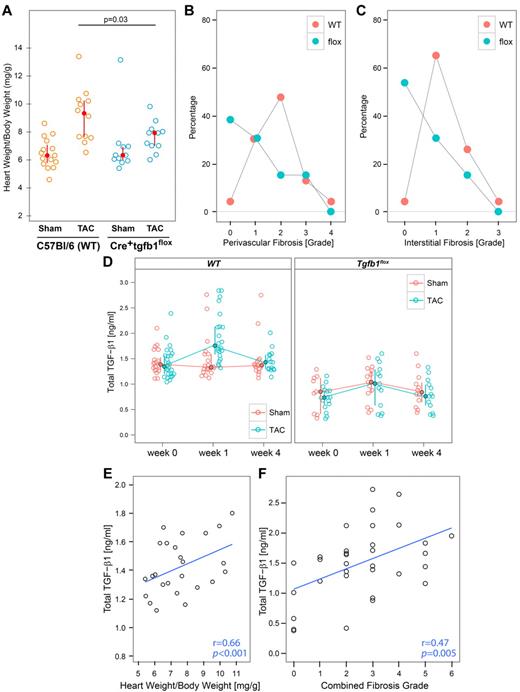
Four weeks after sham surgery, heart weight/body weight ratios were similar in WT and Tgfb1flox mice (Figure 4A; P = .98). In contrast, 4 weeks after TAC surgery, heart weight/body weight ratios were higher in WT mice than Tgfb1flox mice (P = .03), and the values in both types of mice were greater than in the respective sham-treated animals.
Mice with megakaryocyte-specific gene targeting of TGF-β1 are partially protected from developing cardiac hypertrophy and fibrosis 4 weeks after TAC surgery. (A) Heart weight/body weight ratios as calculated by dividing the weight of the perfused heart by total body weight at the time of harvest (4 weeks after the surgery). (B-C) Mice were euthanized and hearts were perfused and fixed in formalin. Heart sections were stained with trichrome and scored for fibrosis by microscopy on a scale from 0 to 4. (B) Perivascular fibrosis scores. (C) Interstitial fibrosis scores. (D) Pretreatment and postsurgery values of plasma TGF-β1 in experimental animals measured by ELISA. (E) The correlations between plasma total TGF-β1 and heart weight/body weight ratio in individual WT mice (r = 0.66; P < .001). (F) The correlation between plasma total TGF-β1 and the combination of interstitial and perivascular fibrosis grade in individual TAC mice of both genotypes.
Mice with megakaryocyte-specific gene targeting of TGF-β1 are partially protected from developing cardiac hypertrophy and fibrosis 4 weeks after TAC surgery. (A) Heart weight/body weight ratios as calculated by dividing the weight of the perfused heart by total body weight at the time of harvest (4 weeks after the surgery). (B-C) Mice were euthanized and hearts were perfused and fixed in formalin. Heart sections were stained with trichrome and scored for fibrosis by microscopy on a scale from 0 to 4. (B) Perivascular fibrosis scores. (C) Interstitial fibrosis scores. (D) Pretreatment and postsurgery values of plasma TGF-β1 in experimental animals measured by ELISA. (E) The correlations between plasma total TGF-β1 and heart weight/body weight ratio in individual WT mice (r = 0.66; P < .001). (F) The correlation between plasma total TGF-β1 and the combination of interstitial and perivascular fibrosis grade in individual TAC mice of both genotypes.
Only a minority of sham-treated WT and Tgfb1flox mice had even mild (1+) interstitial fibrosis 4 weeks after the procedure: 33% (7 of 21) in WT versus 10% (1 of 10) in Tgfb1flox (P = .18). In contrast, 4 weeks after TAC surgery, 96% (22 of 23) of the WT mice had interstitial fibrosis, with 65% (15 of 23) developing mild fibrosis (1+) and 30% (7 of 23) developing moderate (2+) or severe (3+) fibrosis (Figure 4B). Tgfb1flox mice developed less interstitial fibrosis than WT mice, with 54% (7 of 13) having no detectable fibrosis, 31% (4 of 13) having mild (1+) fibrosis, and 15% (2 of 13) having moderate fibrosis (2+); none had severe (3+) fibrosis (Figure 4B; P = .007). The Tgfb1flox mice undergoing TAC also had less perivascular fibrosis than did WT mice, although the differences were less evident (Figure 4C; P = .03). Thus, only 4% (1 of 23) of WT mice had no or minimal perivascular fibrosis, whereas 38% (5 of 13) of Tgfb1flox mice had no or minimal perivascular fibrosis. Representative heart sections stained for fibrosis are shown in supplemental Figure 2. Heart sections were also stained for the presence of myofibroblasts using antibodies to α-smooth muscle actin and vimentin. We found that virtually all of the areas of fibrosis by trichrome staining had cells stained positive for α-smooth muscle actin with or without vimentin, indicating the presence of myofibroblasts31-33 (data not shown).
Tgfb1flox mice have lower plasma levels of TGF-β1 after TAC surgery
Total TGF-β1 plasma levels were higher in WT than Tgfb1flox mice under all conditions (Figure 4D; P < .001). Sham surgery did not affect the plasma levels in the either the WT (P = .59) or Tgfb1flox (P = .29) mice. TAC surgery, however, led to a 0.43-ng/mL (24%) increase in plasma TGF-β1 in WT mice 1 week after surgery (from 1.33 to 1.76 ng/mL; P < .001), which returned to pretreatment values by 4 weeks (Figure 4D). Tgfb1flox mice had a 0.20-ng/mL (22%) increase in plasma TGF-β1 (from 0.71 to 0.91 ng/mL) 1 week after surgery (P = .02), with a return to pretreatment values after 4 weeks. Thus, the ratio of plasma TGF-β1 in Tgfb1flox to WT mice was 0.53 before surgery and 0.52 1 week after surgery.
When the plasma levels of TGF-β1 in individual mice at 1 week were compared with their heart weight/body weight ratios at the end of the experiment, a positive correlation was observed in the WT mice (Figure 4E; r = 0.66, P < .001) but not in Tgfb1flox mice (data not shown; r = −0.11, P = .61). Total TGF-β1 levels 1 week after TAC surgery in both groups of mice also correlated with the combined interstitial and perivascular cardiac fibrosis grades (Figure 4F; r = 0.47; P = .005).
Tgfb1flox mice have better preserved cardiac function after TAC surgery
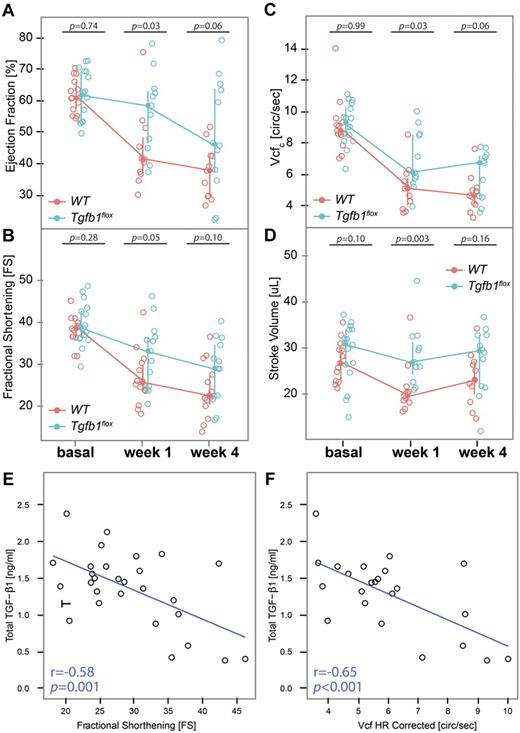
Cardiac function measured by echocardiography showed that WT mice had a 35% reduction in median EF 1 week after TAC surgery (from 64% to 41%), whereas Tgfb1flox mice had only an 11% reduction in EF (from 63% to 56%; P = .03 for Tgfb1flox vs WT; Figure 5A). Other indices of systolic function, including FS, Vcfc, and SV, were also better preserved in Tgfb1flox mice than WT mice after 1 week of TAC surgery (Figures 5B-C and 5D, respectively). Four weeks after TAC surgery, WT mice progressed to a 41% reduction in EF compared with the basal level, whereas Tgfb1flox mice progressed to a 22% reduction (P = .06). FS, Vcfc, and SV were also reduced to a greater extent in WT than Tgfb1flox mice, but the differences were not significant (Figure 5). FS (Figure 5E) and Vcfc (Figure 5F) were inversely correlated with total TGF-β1 1 week after TAC surgery in WT and Tgfb1flox mice (r = −0.58 and −0.65, respectively; P < .001 for both). The indices of diastolic function showed little or no change after TAC surgery in either WT or Tgfb1flox mice (supplemental Table 4).
Mice with megakaryocyte-specific gene targeting of TGF-β1 have better preservation of systolic function than WT mice. (A) EF, (B) FS, (C) Vcfc, and (D) SV were calculated from echocardiographic measurements in WT and Tgfb1flox mice before (basal) and 1 and 4 weeks after TAC surgery. Total TGF-β1 levels at 1 week after surgery were inversely correlated with (E) FS and (F) Vcfc.
Mice with megakaryocyte-specific gene targeting of TGF-β1 have better preservation of systolic function than WT mice. (A) EF, (B) FS, (C) Vcfc, and (D) SV were calculated from echocardiographic measurements in WT and Tgfb1flox mice before (basal) and 1 and 4 weeks after TAC surgery. Total TGF-β1 levels at 1 week after surgery were inversely correlated with (E) FS and (F) Vcfc.
We also compared myocardial gene expression of Col1a1, Col3a1, and Tgfb1 4 weeks after sham or TAC surgery in WT and Tgfb1flox mice (supplemental Figure 3). We found no difference in Col1a1 gene expression between genotypes in either the sham or the TAC groups. There were no differences in Col3a1 gene expression after sham surgery; Col3a1 gene expression was higher in WT mice after TAC versus sham surgery (P = .001). Tgfb1 gene expression was significantly lower after sham surgery in Tgfb1flox mice than WT mice after sham surgery (P < .01). Tgfb1 expression was higher in both groups after TAC surgery, but there was a trend toward lower values in the Tgfb1flox mice (P = .10).
Discussion
The wide range of values of plasma TGF-β1 reported in the literature in health and disease can be explained in large part by the confounding effects of in vitro release of variable amounts of platelet TGF-β1.9 Although several investigators attempted to minimize the impact of in vitro platelet release of TGF-β1, no standardized method of blood drawing and plasma preparation has gained acceptance, nor have quality control measures been proposed to assess the extent of in vitro platelet release. Our data indicate that the standard RB blood drawing technique is associated with variable levels of plasma TGF-β1 resulting from in vitro release of platelet TGF-β1 because adding PGE1 consistently reduces these levels. We also found that increasing the time from blood drawing to plasma preparation was associated with increased in vitro release of TGF-β1. Based on these data, we devised a novel method of obtaining free-flowing blood by percutaneous puncture of the LV under ultrasound guidance and a rapid method of plasma preparation that consistently results in plasma levels in the range of approximately 1.0 ng/mL in mice, which are below those previously reported by most investigators (Table 1). In addition, we analyzed 2 other platelet α-granule proteins, PF4 and TSP-1, as quality control indicators of in vitro platelet release of TGF-β1. We anticipate that these data will help standardize the analysis of plasma TGF-β1 levels.
Reported plasma TGF-β1 values
| Reference . | Strains . | Assays . | Total TGF-β1, ng/mL . |
|---|---|---|---|
| Abdelouahed et al34 | C57Bl/6 | ELISA | 13.4 ± 1.7* |
| 129Sv | 13.0 ± 2.2* | ||
| Evrard et al35 | C57Bl/6 | ELISA | ∼ 6.0-8.0† |
| Beppu et al36 | C57Bl/6 | EIA | ∼ 2.5-3.5† |
| Sanderson et al37 | C57Bl/6 | ELISA | 5.0-37 |
| Kanzler et al38 | C57Bl/6 | ELISA | 5.0 |
| Buday et al39 | C57Bl/6 | ELISA | ∼ 1.6-2.6† |
| Frutkin et al40 | C57Bl/6 | ELISA | ∼ 1.0-3.0‡ |
| Fujimi et al41 | C57Bl/6 | ELISA | ∼ 8.0-12.0† |
| Huang et al42 | ICR/B6D2 | ELISA | ∼ 1.0-1.1† |
| Kopp et al43 | C57Bl/6/CBA | ELISA | 5.6 ± 6.3 |
| Reference . | Strains . | Assays . | Total TGF-β1, ng/mL . |
|---|---|---|---|
| Abdelouahed et al34 | C57Bl/6 | ELISA | 13.4 ± 1.7* |
| 129Sv | 13.0 ± 2.2* | ||
| Evrard et al35 | C57Bl/6 | ELISA | ∼ 6.0-8.0† |
| Beppu et al36 | C57Bl/6 | EIA | ∼ 2.5-3.5† |
| Sanderson et al37 | C57Bl/6 | ELISA | 5.0-37 |
| Kanzler et al38 | C57Bl/6 | ELISA | 5.0 |
| Buday et al39 | C57Bl/6 | ELISA | ∼ 1.6-2.6† |
| Frutkin et al40 | C57Bl/6 | ELISA | ∼ 1.0-3.0‡ |
| Fujimi et al41 | C57Bl/6 | ELISA | ∼ 8.0-12.0† |
| Huang et al42 | ICR/B6D2 | ELISA | ∼ 1.0-1.1† |
| Kopp et al43 | C57Bl/6/CBA | ELISA | 5.6 ± 6.3 |
EIA indicates enzyme immunoassay.
Data are mean ± SEM.
Estimated from graphs of mean value ± SEM or SD.
Estimated from scatter plot.
Our data also suggest an important contribution of platelet-derived TGF-β1 to plasma TGF-β1, independent of in vitro release, because the megakaryocyte-specific gene-targeted mice and the mice rendered severely thrombocytopenic both had lower levels of plasma TGF-β1 than control mice, and the addition of PGE1 to the blood of the Tgfb1flox mice did not further reduce the plasma level of TGF-β1. The percentage reduction in plasma TGF-β1 in the gene-targeted mice was, however, less than the reduction in platelet TGF-β1 (45% vs 85%), suggesting that other cell types may also contribute to plasma TGF-β1.
The studies in which platelets were variably depleted by injecting different doses of the mAb directed at the αIIbβ3 receptor provided important additional insights about the potential in vivo and in vitro contributions of platelets to plasma TGF-β1 levels. At the lowest dose (0.1 mg/kg), platelet counts decreased by approximately 50% in 4 of 5 mice, whereas plasma total TGF-β1 levels were unchanged. This suggests that in vitro release of TGF-β1 during blood drawing and/or plasma preparation using our methods does not make a major contribution to plasma TGF-β1 levels, although other interpretations cannot be excluded (eg, a reduction in in vitro release was precisely matched by an increase in in vivo release, or that plasma TGF-β1 has a long disappearance time in vivo). A similar analysis applies to the relatively stable plasma TGF-β1 levels 24 hours after the 0.25-mg/kg dose, which depleted more than 90% of the platelets. The subsequent increase in plasma TGF-β1 at 48 hours in this group, at a time when the platelet count was still profoundly depressed, could reflect increased in vivo release as a result of platelets undergoing degranulation by participating in hemostasis. A similar explanation could apply to the further increase in TGF-β1 at 72 hours, when the platelet counts were returning to the normal range. The increase over time in plasma TGF-β1 in the mice treated with 0.25 mg/kg contrasts with the approximate 28% decrease in plasma TGF-β1 24 hours after administering the 1.0-mg/kg dose of 1B5. Because there were virtually no circulating platelets to release TGF-β1 in vitro, these data further support a minimal role for in vitro release of platelet TGF-β1 when using our blood drawing and plasma preparation techniques. One possible explanation for the paradoxical decrease in total TGF-β1 values with the 1.0-mg/kg dose relative to the increase with the 0.25-mg/kg dose is that the higher dose produced more profound thrombocytopenia, thus limiting the number of platelets available to contribute to enhanced in vivo release of platelet TGF-β1 during hemostasis. Without this enhanced release, this dose unmasks the effect of the reduced number of platelets available to maintain the steady-state levels of plasma TGF-β1 through in vivo release. The expected rate of decrease in plasma TGF-β1 in the absence of ongoing in vivo release of TGF-β1 from platelets depends on the plasma disappearance rate of TGF-β1, for which we do not have any direct measurement. However, because we did observe a decrease in the mice given the highest dose, we infer that the plasma disappearance rate is of the order of minutes to hours, rather than days or weeks which is consistent with the report by Wakefield et al.44
Our data demonstrating basal differences in left ventricular mass and arterial diameter between Tgfb1flox and WT mice raise the possibility that platelet TGF-β1 may have a subtle role in cardiovascular development that has not previously been appreciated. Indeed, a relationship between aortic abnormalities and TGF-β1 activity has been proposed to contribute to the aneurysms found in Marfan syndrome, which is the result of defects in the arterial wall protein fibrillin.45-47 Further studies are needed to assess the role of platelet TGF-β1 in vascular development and biology.
Mice with megakaryocyte-specific deletion of TGF-β1 had less cardiac interstitial and perivascular fibrosis than WT mice after TAC surgery. This correlated with the presence of fewer myofibroblasts in the hearts of Tgfb1flox than WT mice, with the myofibroblasts enriched in the areas of fibrosis. Because TGF-β1 is known to induce myofibroblast differentiation,31-33 these data are consistent with a role for platelet TGF-β1 in initiating fibrosis. However, because other agents are also capable of inducing myofibroblast differentiation,47-49 they do not unequivocally establish that TGF-β1 was responsible for the transformation. Tgfβ1 mice also had lower heart weight/body weight ratios compared with WT mice and better preserved cardiac systolic function. Together, these data support a role for platelet TGF-β1 in cardiac hypertrophy and fibrosis in response to pressure overload. This hypothesis is further supported by the association between plasma TGF-β1 levels and both heart weight/body weight ratios, fibrosis, and systolic function.
Because the Tgfb1flox mice had larger innominate arteries before the TAC surgery, we wondered whether some or all of the protection against cardiac hypertrophy and fibrosis resulted from a greater capacity of the innominate artery to accommodate the increase in blood flow produced by the TAC stenosis. We judged this unlikely, however, because one week after TAC surgery there were no significant differences in the size of the innominate arteries, the mean and peak blood flow velocities in the stenoses, or the pressure gradients across the stenoses of WT and Tgfb1flox mice. The protection from fibrosis in the Tgfb1flox mice was not complete, and this may be explained by the residual platelet TGF-β1 (∼ 15%), the mechanical effects of pressure overload independent of TGF-β1, and/or contributions of TGF-β1 and/or other fibrogenic agents produced by other cells.50
In conclusion: (1) accurate measurements of plasma TGF-β1 can be made in mice, but they require special blood collection and plasma preparation techniques to avoid in vitro release of platelet TGF-β1; (2) platelets contribute to plasma levels of TGF-β1, presumably via in vivo release; and (3) platelet TGF-β1 may contribute to cardiac hypertrophy, fibrosis, and systolic dysfunction induced by the TAC model of pressure overload. Collectively, these data provide both a conceptual and practical framework for analyzing plasma TGF-β1 in mouse models of human disease and for more detailed studies of the role of platelet-derived TGF-β1 in wide spectrum of physiologic and pathologic processes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Note added in proof:
After our manuscript was accepted for publication, Labell et al reported on another transgenic mouse with targeted deletion of megakargocyte and platelet TGF-β1.52 They independently conclude that platelet TGF-β1 contributes to plasma TGF-β1 but that there are other sources of plasma TGF-β1.
Acknowledgments
The authors thank Dr Daniel Rifkin for kindly providing the mink lung epithelial cells, Dr Teresa Brophy for valuable discussion, Dr Marketa Jirouskova for performing the FeCl3 thrombosis model, Spandana Vootukuri and Jihong Li for technical support, Dr Skye Rasmussen for assistance with mouse surgery, Suzanne Rivera for administrative assistance, Tingting Song for statistical analysis, and Drs Gopinath Palanisamy and Virginia Gillespie for cardiac fibrosis analysis and scoring.
This work was supported in part by a New York City Community Trust Grant Award for Young Faculty (J.A.), an Irma-Hirschl/Monique Weill-Caulier Trust Research Award (J.A.), the National Center for Research Resources (National Institutes of Health; Clinical and Translational Science Award 5UL1RR024143), the National Heart, Lung, and Blood Institute (grant HL085357; J.L.D.), a German National Academic Foundation Grant (A.M.), and Stony Brook University.
National Institutes of Health
Authorship
Contribution: A.M. and W.W. performed experiments and interpreted results; J.Q. performed select experiments; L.C. advised and assisted with the echocardiographic measurements and data interpretation; J.L.D. generated the PF4Cre+/+Tgfb1flox/flox mice; B.S.C. designed experiments, interpreted results, and assisted in writing the paper; and J.A. designed and performed experiments, interpreted results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jasimuddin Ahamed, Laboratory of Blood and Vascular Biology, Rockefeller University, 1230 York Ave, New York, NY 10065; e-mail: jahamed@rockefeller.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal