Key Points
The first embryonic platelets are produced by a unique lineage of diploid cells not polyploid MKs.
Diploid platelet-forming cells are produced in the early mouse embryo via a progenitor cell–independent pathway.
Abstract
In this study, we test the assumption that the hematopoietic progenitor/colony-forming cells of the embryonic yolk sac (YS), which are endowed with megakaryocytic potential, differentiate into the first platelet-forming cells in vivo. We demonstrate that from embryonic day (E) 8.5 all megakaryocyte (MK) colony-forming cells belong to the conventional hematopoietic progenitor cell (HPC) compartment. Although these cells are indeed capable of generating polyploid MKs, they are not the source of the first platelet-forming cells. We show that proplatelet formation first occurs in a unique and previously unrecognized lineage of diploid platelet-forming cells, which develop within the YS in parallel to HPCs but can be specified in the E8.5 Runx1-null embryo despite the absence of the progenitor cell lineage.
Introduction
Blood cell formation first occurs within the embryonic yolk sac (YS), yielding primitive red cells and multiple classes of colony-forming units (CFUs)/hematopoietic progenitor cells (HPCs).1 From embryonic day (E) 7.5, the YS contains megakaryocyte (MK) CFUs2-4 and, by E8.5, is capable of generating polyploid MKs in vitro.2 Detection of circulating platelets soon follows the appearance of HPCs.4 The intuitive extrapolation is that MK-CFUs differentiate within the YS to initiate platelet formation, but this model remains untested.
To better understand how platelet production proceeds in the YS, we have employed a combination of functional assays, transcriptomics, imaging, and gene disruption to investigate the relationship between HPCs and generation of the first platelet-forming cells.
Study design
UBI-gfp,5 Runx1LacZ/LacZ,6 and Runx1Δ/Δ7 mouse lines were maintained as C57BL/6. Walter and Eliza Hall Institute Animal Ethics Committee approved the experiments. Developmental stages were determined morphologically or by counting somite pairs.
For CFU-MK culture, MegaCult-C (StemCell Technologies) was used as previously described8 ; colonies were defined as clusters of ≥10 CD41+ cells after 5 days. M3434 (StemCell Technologies) was used for myelo-erythroid assays.
Proplatelet assays were performed using serum-free medium.9 Time-lapse imaging was performed using microgrid arrays (Microsurfaces) attached to coverslip chamber slides.10
An LSM 780 microscope was used for confocal imaging. Analysis was performed using Imaris (Bitplane).
For transcriptome analyses, samples were hybridized to Illumina Expression BeadChips (ArrayExpress accession: E-MTAB-2625) and analyzed using limma.
Results and discussion
Although MK-CFUs have been functionally identified in the YS,2,4 the immunophenotype of these progenitors is undescribed, making it unclear how they relate to the conventional CD45+CD41low HPC population.11,12 To address this, we isolated cells expressing CD45 and/or CD41 from E9.5 and E10.5 YS (Figure 1A) and performed in vitro colony-forming assays. At both stages, all MK-CFUs present in whole YS were accounted for by the conventional HPC population (Figure 1B; supplemental Figure 1, see the Blood Web site).
Identification of diploid platelet-forming cells (DPFCs) in the E10.5 YS. (A) CD45 and CD41 expression in pooled E10.5 YS. Values indicate the numbers (mean ± standard error of the mean [SEM]) of each population per embryo equivalent (n = 15). (B) Distribution of CFUs with MK potential in 1 embryo equivalent of whole or purified E9.5 and E10.5 YSs. Cells were dissociated in 10% collagenase/dispase for 45 minutes at 37°C and then dissociated mechanically. No CD45+CD41− cells were present in E9.5 YS. Error bars represent SEM (n = 3). sp, somite pairs. (C) To distinguish embryonic from contaminating maternal platelets in preparations of embryonic peripheral blood, green fluorescent protein (GFP)–expressing male mice were mated with wild-type females, ensuring that all GFP+ cells were of embryonic origin. Flow cytometry plots showing the presence of GFP+ platelets in the PB of E9.5 (i) and E10.5 (ii) embryos. (iii) Quantification of embryonic platelets in the PB of E9.5–10.5 embryos. Error bars represent standard deviation (SD) (n = 9–21 embryos per stage). (D) (i) Representative image of E10.5 YS CD45+CD41low HPCs cultured in proplatelet medium (including anti-CD41-APC) for 72 hours (n = 5). Red indicates CD41 expression. Scale bar represents 40 μm. (ii) DNA content analysis of HPC-derived CD41highCD42D+ cells demonstrating a conventional MK 2–16n ploidy profile (n = 3). Following antibody staining, cells were fixed in 80% ethanol, permeabilized in TritonX-100, and stained with 4,6-diamidino-2-phenylindole (DAPI). (E) Multidimensional scaling plot of microarray data comparing transcriptional similarity of E10.5 YS CD45−CD41high cells with E10.5 YS lineages (HPC = CD45+CD41low; myeloid = CD45+CD41−; endothelial = VECAD+CD45−CD41−) and E13.5 fetal liver (FL) lineages (blast = CD45+CD11B−; myeloid = CD45+CD11B+; and MK = CD41highCD42D+CD150+). Note that myeloid and HPC populations from the YS cluster with their FL counterparts, whereas YS CD45−CD41high cells cluster most strongly with E13.5 FL MKs. (F) DNA content analysis of E10.5 YS CD45−CD41high cells demonstrating that these cells are predominantly 2n in vivo (n = 3). (G) Cumulative frequency plot of in vitro proplatelet-forming capacity of 145 CD45−CD41high pedigrees. Cells were cultured in serum-free medium with 100 ng/mL recombinant mouse thrombopoietin (THPO) (including anti-CD41-APC) and live-imaged at a 3- to 4-minute time resolution. Data from 3 independent experiments are shown. For each experiment, 6000 cells were plated per chamber. The number of microwells imaged was limited by the number of positions that could be imaged within a 3- to 4-minute time period. n = number of pedigrees per experiment. (H) Representative confocal z-stack of E10.5 YS CD45−CD41high cells after 72 hours in serum-free medium with 100 ng/mL recombinant mouse THPO (n = 30). Cultures were fixed in 2% paraformaldehyde (PFA) for 20 minutes, permeabilized with 0.6% TritonX/10% fetal calf serum (FCS), and stained with anti-CD41 and anti-CD42D antibodies (0.3% TritonX/5% FCS); nuclei were stained with DAPI. Note that cells are generating proplatelets while in a diploid state. Scale bar represents 10 μm. (I) Scatter plot of object volumes and relative DNA content of CD41highCD42D+ cells within E10.5 YSs that are in the process of platelet production in vivo. Surface objects for nuclei were generated by manually defining the area of DAPI staining within individual cells using Imaris software (Bitplane); intensity sum values representing the 4n state were generated from mitotic figures from CD41−CD42D− cells within the z-stack. Relative DNA content was derived by dividing the intensity sum of CD41highCD42D+ nuclei by that of a mitotic figure within the same z-stack. This strategy allows determination of ploidy of cells within the z-stack that are, or are not, in the process of platelet production. A value of 0.5 represents 2n, and 1.0 represents 4n. Data are derived from 10 individual z-stacks taken from 5 YSs. (J) Representative confocal z-stack of CD41highCD42D+ cells within the vascular endothelial cadherin (VECAD)–expressing vasculature of the E10.5 YS in vivo. Surface objects of DNA (DAPI) content are overlaid and reflect the range of DNA content of CD41highCD42D+ cells in vivo. The diploid state of a proplatelet-forming cell (blue arrowhead) is highlighted. Freshly dissected YSs were fixed in 2% PFA for 20 minutes, permeabilized with 0.6% TritonX/10% FCS for 30 minutes, and stained with anti-CD41, anti-VECAD, and anti-CD42D antibodies (0.3% TritonX/5% FCS); nuclei were stained with DAPI. Colors reflect the intensity sum of DAPI signal, ranging from the 2n (blue) to 4n (red) states. Scale bar represents 10 μm; n = 20.
Identification of diploid platelet-forming cells (DPFCs) in the E10.5 YS. (A) CD45 and CD41 expression in pooled E10.5 YS. Values indicate the numbers (mean ± standard error of the mean [SEM]) of each population per embryo equivalent (n = 15). (B) Distribution of CFUs with MK potential in 1 embryo equivalent of whole or purified E9.5 and E10.5 YSs. Cells were dissociated in 10% collagenase/dispase for 45 minutes at 37°C and then dissociated mechanically. No CD45+CD41− cells were present in E9.5 YS. Error bars represent SEM (n = 3). sp, somite pairs. (C) To distinguish embryonic from contaminating maternal platelets in preparations of embryonic peripheral blood, green fluorescent protein (GFP)–expressing male mice were mated with wild-type females, ensuring that all GFP+ cells were of embryonic origin. Flow cytometry plots showing the presence of GFP+ platelets in the PB of E9.5 (i) and E10.5 (ii) embryos. (iii) Quantification of embryonic platelets in the PB of E9.5–10.5 embryos. Error bars represent standard deviation (SD) (n = 9–21 embryos per stage). (D) (i) Representative image of E10.5 YS CD45+CD41low HPCs cultured in proplatelet medium (including anti-CD41-APC) for 72 hours (n = 5). Red indicates CD41 expression. Scale bar represents 40 μm. (ii) DNA content analysis of HPC-derived CD41highCD42D+ cells demonstrating a conventional MK 2–16n ploidy profile (n = 3). Following antibody staining, cells were fixed in 80% ethanol, permeabilized in TritonX-100, and stained with 4,6-diamidino-2-phenylindole (DAPI). (E) Multidimensional scaling plot of microarray data comparing transcriptional similarity of E10.5 YS CD45−CD41high cells with E10.5 YS lineages (HPC = CD45+CD41low; myeloid = CD45+CD41−; endothelial = VECAD+CD45−CD41−) and E13.5 fetal liver (FL) lineages (blast = CD45+CD11B−; myeloid = CD45+CD11B+; and MK = CD41highCD42D+CD150+). Note that myeloid and HPC populations from the YS cluster with their FL counterparts, whereas YS CD45−CD41high cells cluster most strongly with E13.5 FL MKs. (F) DNA content analysis of E10.5 YS CD45−CD41high cells demonstrating that these cells are predominantly 2n in vivo (n = 3). (G) Cumulative frequency plot of in vitro proplatelet-forming capacity of 145 CD45−CD41high pedigrees. Cells were cultured in serum-free medium with 100 ng/mL recombinant mouse thrombopoietin (THPO) (including anti-CD41-APC) and live-imaged at a 3- to 4-minute time resolution. Data from 3 independent experiments are shown. For each experiment, 6000 cells were plated per chamber. The number of microwells imaged was limited by the number of positions that could be imaged within a 3- to 4-minute time period. n = number of pedigrees per experiment. (H) Representative confocal z-stack of E10.5 YS CD45−CD41high cells after 72 hours in serum-free medium with 100 ng/mL recombinant mouse THPO (n = 30). Cultures were fixed in 2% paraformaldehyde (PFA) for 20 minutes, permeabilized with 0.6% TritonX/10% fetal calf serum (FCS), and stained with anti-CD41 and anti-CD42D antibodies (0.3% TritonX/5% FCS); nuclei were stained with DAPI. Note that cells are generating proplatelets while in a diploid state. Scale bar represents 10 μm. (I) Scatter plot of object volumes and relative DNA content of CD41highCD42D+ cells within E10.5 YSs that are in the process of platelet production in vivo. Surface objects for nuclei were generated by manually defining the area of DAPI staining within individual cells using Imaris software (Bitplane); intensity sum values representing the 4n state were generated from mitotic figures from CD41−CD42D− cells within the z-stack. Relative DNA content was derived by dividing the intensity sum of CD41highCD42D+ nuclei by that of a mitotic figure within the same z-stack. This strategy allows determination of ploidy of cells within the z-stack that are, or are not, in the process of platelet production. A value of 0.5 represents 2n, and 1.0 represents 4n. Data are derived from 10 individual z-stacks taken from 5 YSs. (J) Representative confocal z-stack of CD41highCD42D+ cells within the vascular endothelial cadherin (VECAD)–expressing vasculature of the E10.5 YS in vivo. Surface objects of DNA (DAPI) content are overlaid and reflect the range of DNA content of CD41highCD42D+ cells in vivo. The diploid state of a proplatelet-forming cell (blue arrowhead) is highlighted. Freshly dissected YSs were fixed in 2% PFA for 20 minutes, permeabilized with 0.6% TritonX/10% FCS for 30 minutes, and stained with anti-CD41, anti-VECAD, and anti-CD42D antibodies (0.3% TritonX/5% FCS); nuclei were stained with DAPI. Colors reflect the intensity sum of DAPI signal, ranging from the 2n (blue) to 4n (red) states. Scale bar represents 10 μm; n = 20.
We found that platelets first entered the peripheral blood (PB) from E9.5, increasing rapidly in number by E10.5 (Figure 1C). It would therefore be expected that the cells responsible for platelet production are terminally differentiated by E10.5. Hallmarks of mature MKs are their highly polyploid nuclei and the ability to form proplatelets.13 Proplatelets can be acutely induced ex vivo from MKs within hours,14 whereas production of proplatelet-forming MKs from fetal precursors require at least 4 days.15 We found that after 72 hours in vitro HPCs generated polyploid MKs, but proplatelets were rarely formed (Figure 1D), indicating that HPCs had not differentiated into acute proplatelet-forming cells in vivo and were therefore not the source of the first platelets.
We next queried if an alternative population was responsible for platelet formation. By comparing the transcriptional profiles of E10.5 YS cells with E13.5 liver reference lineages, including MKs, we investigated if elements of an MK signature were present at E10.5. We found that a previously uncharacterized population of CD45−CD41high cells was strikingly similar to E13.5 MKs (Figure 1E, supplemental Figure 2A); this population also coexpressed MK-associated proteins including MPL (Myeloproliferative leukemia virus oncogene), CD42D, and acetylcholinesterase (supplemental Figure 2B-C). Curiously, CD45−CD41high cells did not exhibit the high ploidy range associated with conventional MKs or E10.5 YS HPC-derived MKs; rather, the majority were diploid (Figure 1F).
Approximately half of the low-ploidy CD45−CD41high cells acutely produced proplatelets in vitro (Figure 1G). Similarly to FL MKs,16 in vitro thrombopoiesis was not THPO dependent (supplemental Figure 2D), but surprisingly, proplatelets were formed while in a diploid state (Figure 1H).
Using a refined MK immunophenotype (CD41highCD42D+), we confirmed that proplatelet formation also occurred in a diploid state in vivo (Figure 1I-J). We therefore defined this lineage as DPFCs.
That HPC-derived MKs were highly polyploid yet in vivo platelet-forming cells were diploid prompted us to ask whether HPCs are the source of YS DPFCs in vivo. To address this, we investigated when DPFC commitment first occurred.
CD41highCD42D+ cells were generated in vitro from E7.5 and E7.75 YSs, but acute proplatelet formation was rarely observed, and only from E7.75 (Figure 2A). By E8.5 CD41low/−TER119+ primitive erythroid (EryP)11 and VECAD+CD41high HPC lineages can be prospectively isolated.11,17 Accompanying these, we identified a population of VECAD−CD41high cells that encompassed all CD42D+ cells (Figure 2B), indicating that they might include acute proplatelet-forming cells.
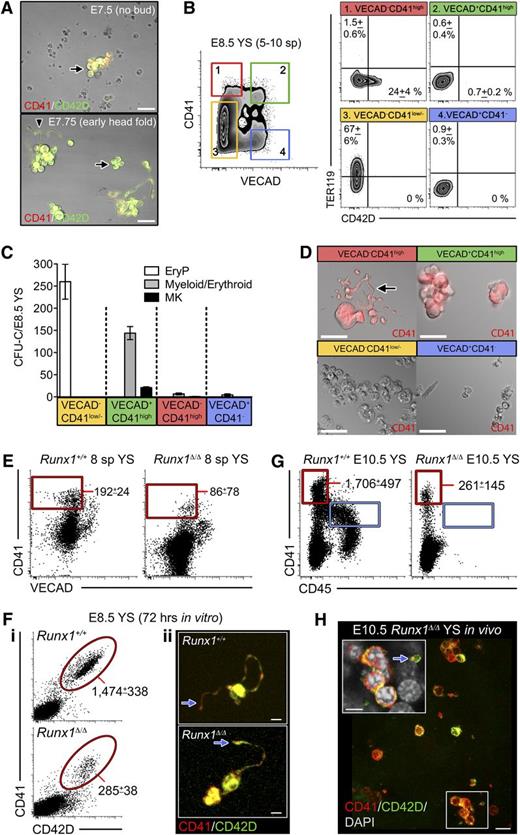
DPFCs emerge in parallel to the progenitor cell lineage. (A) Representative image of E7.5 and E7.75 YS cultures after 72 hours. Scale bar represents 20 μm. After dissection, YSs were incubated in 0.25% trypsin/EDTA at 37°C for 5 minutes, mechanically dissociated, and then cultured in serum-free medium with 100 ng/mL recombinant mouse THPO (anti-CD41-APC and anti-CD42D-PE antibodies were added to the culture medium). Note that CD41+CD42D+ cells (arrows) were generated from both stages, but proplatelets (arrowhead) were only from E7.75 cultures (n = 3). Scale bar represents 30 μm. (B) Plots of VECAD, CD41, CD42D, and TER119 expression in pooled E8.5 YSs (n = 3). (C) Distribution of E8.5 CFU-EryP (white), CFU-myeloid/erythroid (gray), and CFU-MK (black) in 1 embryo equivalent of sorted YS cells. Error bars indicate SEM (n = 3). EryP colonies were distinguished from erythroid burst-forming unit (BFU-E; which contain both adult-type red cells and 1 leukocyte lineage) according to morphology. (D) Representative images from cultures of purified E8.5 YS populations cultured in proplatelet medium (with anti-CD41-APC and anti-CD42D-PE) for 72 hours (n = 3). Scale bar represents 30 μm. Although VECAD−CD41high cells generated CD41+CD42D+ MKs, proplatelet-forming CD41+CD42+ cells (arrow) were only observed from cultures of VECAD−CD41high cells. (E) Representative plots from Runx1+/+ (n = 5) and Runx1Δ/Δ (n = 6) E8.5 YS cells at the 8-sp stage demonstrating that the VECAD−CD41high proplatelet-forming lineage is specified in the absence of RUNX1. Values indicate the numbers of cells detected (mean ± SD). (F) E8.5 Runx1Δ/Δ YSs were capable of forming CD41+CD42D+ cells after 72 hours in the proplatelet assay (i); these cells are capable of proplatelet formation in vitro (ii). Values represent the mean ± SEM (n = 3). Scale bar represents 30 μm. (G) Representative plot of CD45 and CD41 expression in E10.5 YS from Runx1+/+ (n = 6) and Runx1-null (n = 8) embryos demonstrating that in the absence of RUNX1 DPFCs can develop (red gate) while HPCs are completely absent (blue gate). Values indicate the numbers of cells detected (mean ± SD). (H) Representative (n = 6) confocal z-stack of Runx1Δ/Δ E10.5 YS showing CD41highCD42D+ DPFCs and platelets (arrow) can be produced in vivo despite the complete absence of HPCs. Scale bar represents 10 μm. Inset, optical section of boxed region showing a free platelet (arrow) within the Runx1-null YS. Scale bar represents 5 μm.
DPFCs emerge in parallel to the progenitor cell lineage. (A) Representative image of E7.5 and E7.75 YS cultures after 72 hours. Scale bar represents 20 μm. After dissection, YSs were incubated in 0.25% trypsin/EDTA at 37°C for 5 minutes, mechanically dissociated, and then cultured in serum-free medium with 100 ng/mL recombinant mouse THPO (anti-CD41-APC and anti-CD42D-PE antibodies were added to the culture medium). Note that CD41+CD42D+ cells (arrows) were generated from both stages, but proplatelets (arrowhead) were only from E7.75 cultures (n = 3). Scale bar represents 30 μm. (B) Plots of VECAD, CD41, CD42D, and TER119 expression in pooled E8.5 YSs (n = 3). (C) Distribution of E8.5 CFU-EryP (white), CFU-myeloid/erythroid (gray), and CFU-MK (black) in 1 embryo equivalent of sorted YS cells. Error bars indicate SEM (n = 3). EryP colonies were distinguished from erythroid burst-forming unit (BFU-E; which contain both adult-type red cells and 1 leukocyte lineage) according to morphology. (D) Representative images from cultures of purified E8.5 YS populations cultured in proplatelet medium (with anti-CD41-APC and anti-CD42D-PE) for 72 hours (n = 3). Scale bar represents 30 μm. Although VECAD−CD41high cells generated CD41+CD42D+ MKs, proplatelet-forming CD41+CD42+ cells (arrow) were only observed from cultures of VECAD−CD41high cells. (E) Representative plots from Runx1+/+ (n = 5) and Runx1Δ/Δ (n = 6) E8.5 YS cells at the 8-sp stage demonstrating that the VECAD−CD41high proplatelet-forming lineage is specified in the absence of RUNX1. Values indicate the numbers of cells detected (mean ± SD). (F) E8.5 Runx1Δ/Δ YSs were capable of forming CD41+CD42D+ cells after 72 hours in the proplatelet assay (i); these cells are capable of proplatelet formation in vitro (ii). Values represent the mean ± SEM (n = 3). Scale bar represents 30 μm. (G) Representative plot of CD45 and CD41 expression in E10.5 YS from Runx1+/+ (n = 6) and Runx1-null (n = 8) embryos demonstrating that in the absence of RUNX1 DPFCs can develop (red gate) while HPCs are completely absent (blue gate). Values indicate the numbers of cells detected (mean ± SD). (H) Representative (n = 6) confocal z-stack of Runx1Δ/Δ E10.5 YS showing CD41highCD42D+ DPFCs and platelets (arrow) can be produced in vivo despite the complete absence of HPCs. Scale bar represents 10 μm. Inset, optical section of boxed region showing a free platelet (arrow) within the Runx1-null YS. Scale bar represents 5 μm.
CFU and proplatelet assays revealed that VECAD+CD41high cells contained all myeloid/erythroid and MK CFUs, but only VECAD−CD41high cells were capable of acute proplatelet formation (Figure 2C-D, supplemental Figure 3). That E8.5 VECAD−CD41high cells displayed little acetylcholinesterase activity (supplemental Figure 4) suggested that they are an immature DPFC precursor. Thus, as early as E8.5, acute proplatelet-forming cells exist in the YS and are immunophenotypically distinct from the HPC lineage.
Hematopoietic commitment in the YS occurs via VECAD-expressing precursors.18,19 Consistent with this, all CD41-expressing cells in the E7.75–8.25 blood band coexpressed VECAD, but by E8.5, they had diverged into VECAD+ (HPC) and VECAD− (pre-DPFC) counterparts (supplemental Figure 5). This suggested that HPC and DPFC lineages both progressed via VECAD-expressing precursors. We questioned whether YS DPFCs arise in parallel to, but independently of, HPCs.
Determining whether a lineage is HPC derived in the YS has been problematic. The best attempts have used c-Myb−/− mice.3,20 However, multiple classes of HPCs are produced in c-Myb−/− embryos; among these are erythroid, macrophage, and MK CFUs.3,21 We reasoned that a more stringent approach would be the use of Runx1-null embryos. Without RUNX1, HPC formation is completely blocked22,23 ; yet hematopoietic specification from the mesoderm is permitted, as evidenced by EryP formation.24 Thus, studying the Runx1-null YS should allow us to test if HPCs give rise to DPFCs.
Using 2 independent Runx1-null lines6,7 (both carrying the null allele through the germ line), we found that the E8.5 Runx1-null YSs contained VECAD−CD41high pre-DPFCs and were capable of generating CD41+CD42D+ cells in vitro (Figure 2E-F). Analysis of E10.5 Runx1-null YS confirmed that despite the absence of HPCs, platelet-producing DPFCs were formed in vivo (Figure 2G-H). Although Runx1-null DPFC numbers were lower both in vitro and in vivo, which is likely a result of RUNX1 being an essential factor for megakaryopoiesis,25 these proof-of-concept experiments demonstrated that developmental specification of the DPFC lineage was not HPC dependent.
In summary, we have shown that polyploid MKs generated from YS cultures,2 which resemble MKs of fetal life, are the product of HPC differentiation. These do not represent the first in vivo platelet-forming cells of the embryo. Platelet formation in the YS is initiated by a previously unrecognized cell that we have termed DPFC, which likely develops via the primitive hematopoietic pathway.2,4 A key challenge will now be to experimentally define when HPC-derived conventional MKs supersede DPFCs to become the source of platelets during fetal life.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Julie Sheridan for critical discussions.
This work was supported by the Australian Research Council (Special Research Initiative in Stem Cell Sciences, Discovery Early Career Researcher Award Fellowship [S.T.], Strategic Australian Postgraduate Award Studentship [K.S.P.]), Program Grant (1016647), Fellowships (W.S.A., G.K.S., and D.J.H.), and Independent Research Institutes Infrastructure Support Scheme Grant (361646) from the National Health and Medical Research Council, and Victorian State Government Operational Infrastructure Support.
Authorship
Contribution: K.S.P., S.T., W.S.A., and D.J.H. designed the research, analyzed data, and wrote the manuscript; J.F.M., C.B., E.C.J., L.W.W., K.L.R., A.L., B.T.K., and A.M. designed the research; and T.J.S., W.S., and G.K.S. analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Samir Taoudi, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Melbourne, VIC 3052, Australia; e-mail: taoudi@wehi.edu.au.
References
Author notes
D.J.H. and S.T. contributed equally to this study.

![Figure 1. Identification of diploid platelet-forming cells (DPFCs) in the E10.5 YS. (A) CD45 and CD41 expression in pooled E10.5 YS. Values indicate the numbers (mean ± standard error of the mean [SEM]) of each population per embryo equivalent (n = 15). (B) Distribution of CFUs with MK potential in 1 embryo equivalent of whole or purified E9.5 and E10.5 YSs. Cells were dissociated in 10% collagenase/dispase for 45 minutes at 37°C and then dissociated mechanically. No CD45+CD41− cells were present in E9.5 YS. Error bars represent SEM (n = 3). sp, somite pairs. (C) To distinguish embryonic from contaminating maternal platelets in preparations of embryonic peripheral blood, green fluorescent protein (GFP)–expressing male mice were mated with wild-type females, ensuring that all GFP+ cells were of embryonic origin. Flow cytometry plots showing the presence of GFP+ platelets in the PB of E9.5 (i) and E10.5 (ii) embryos. (iii) Quantification of embryonic platelets in the PB of E9.5–10.5 embryos. Error bars represent standard deviation (SD) (n = 9–21 embryos per stage). (D) (i) Representative image of E10.5 YS CD45+CD41low HPCs cultured in proplatelet medium (including anti-CD41-APC) for 72 hours (n = 5). Red indicates CD41 expression. Scale bar represents 40 μm. (ii) DNA content analysis of HPC-derived CD41highCD42D+ cells demonstrating a conventional MK 2–16n ploidy profile (n = 3). Following antibody staining, cells were fixed in 80% ethanol, permeabilized in TritonX-100, and stained with 4,6-diamidino-2-phenylindole (DAPI). (E) Multidimensional scaling plot of microarray data comparing transcriptional similarity of E10.5 YS CD45−CD41high cells with E10.5 YS lineages (HPC = CD45+CD41low; myeloid = CD45+CD41−; endothelial = VECAD+CD45−CD41−) and E13.5 fetal liver (FL) lineages (blast = CD45+CD11B−; myeloid = CD45+CD11B+; and MK = CD41highCD42D+CD150+). Note that myeloid and HPC populations from the YS cluster with their FL counterparts, whereas YS CD45−CD41high cells cluster most strongly with E13.5 FL MKs. (F) DNA content analysis of E10.5 YS CD45−CD41high cells demonstrating that these cells are predominantly 2n in vivo (n = 3). (G) Cumulative frequency plot of in vitro proplatelet-forming capacity of 145 CD45−CD41high pedigrees. Cells were cultured in serum-free medium with 100 ng/mL recombinant mouse thrombopoietin (THPO) (including anti-CD41-APC) and live-imaged at a 3- to 4-minute time resolution. Data from 3 independent experiments are shown. For each experiment, 6000 cells were plated per chamber. The number of microwells imaged was limited by the number of positions that could be imaged within a 3- to 4-minute time period. n = number of pedigrees per experiment. (H) Representative confocal z-stack of E10.5 YS CD45−CD41high cells after 72 hours in serum-free medium with 100 ng/mL recombinant mouse THPO (n = 30). Cultures were fixed in 2% paraformaldehyde (PFA) for 20 minutes, permeabilized with 0.6% TritonX/10% fetal calf serum (FCS), and stained with anti-CD41 and anti-CD42D antibodies (0.3% TritonX/5% FCS); nuclei were stained with DAPI. Note that cells are generating proplatelets while in a diploid state. Scale bar represents 10 μm. (I) Scatter plot of object volumes and relative DNA content of CD41highCD42D+ cells within E10.5 YSs that are in the process of platelet production in vivo. Surface objects for nuclei were generated by manually defining the area of DAPI staining within individual cells using Imaris software (Bitplane); intensity sum values representing the 4n state were generated from mitotic figures from CD41−CD42D− cells within the z-stack. Relative DNA content was derived by dividing the intensity sum of CD41highCD42D+ nuclei by that of a mitotic figure within the same z-stack. This strategy allows determination of ploidy of cells within the z-stack that are, or are not, in the process of platelet production. A value of 0.5 represents 2n, and 1.0 represents 4n. Data are derived from 10 individual z-stacks taken from 5 YSs. (J) Representative confocal z-stack of CD41highCD42D+ cells within the vascular endothelial cadherin (VECAD)–expressing vasculature of the E10.5 YS in vivo. Surface objects of DNA (DAPI) content are overlaid and reflect the range of DNA content of CD41highCD42D+ cells in vivo. The diploid state of a proplatelet-forming cell (blue arrowhead) is highlighted. Freshly dissected YSs were fixed in 2% PFA for 20 minutes, permeabilized with 0.6% TritonX/10% FCS for 30 minutes, and stained with anti-CD41, anti-VECAD, and anti-CD42D antibodies (0.3% TritonX/5% FCS); nuclei were stained with DAPI. Colors reflect the intensity sum of DAPI signal, ranging from the 2n (blue) to 4n (red) states. Scale bar represents 10 μm; n = 20.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/17/10.1182_blood-2014-02-559468/4/m_2725f1.jpeg?Expires=1767724803&Signature=3piUHUlE2whxeTI9w-Zq3azCuZXq97~N4XhDF~1aoQT0bKsMFw5ZXGMSinskkTH448YVq8CGQx70wbN0dYJl-mQOa3UQnGGT~EGbZovTfJPzLI72ay~d1JJDKyVDjcltuoRWnoNwlR31u8kKREqeAKfjUlvYefwn8hKy5sIR9FbWkHgJ-Zosl1YoNgimZvpSQ69wEIqDUVaCjJIghYxEuD~VZsPuMZQ2tV53G2XbXQwk9rUGZ2WxyP5ofzKP4tlR6KbQMaEQtwAw-EBXlnQXKkZloS7LpzZzEeR5uqTWbEKLEW6t7lJLTZfIO7C8BstMy7kWDxuTAbPW3JDm8HkEaA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal