Abstract
Introduction
FLT3-ITD (Internal tandem duplication) and FLT3-TKD (tyrosine kinase domain, D835) mutations are frequently seen in AML; FLT3-ITDs are associated with inferior survival. Development of FLT3-TKD mutation during FLT3 inhibitor-therapy is seen in up to 22% of patients and associated with FLT3 tyrosine kinase inhibitor (TKI) treatment failure (Cancer 2014). Crenolanib besylate is an orally bioavailable TKI with activity against both FLT3-ITD and FLT3-TKD mutations. We evaluated the clinical efficacy of crenolanib in relapsed/refractory AML pts with activating FLT3 mutations.
Methods
This was a single center phase II open label study of crenolanib administered at 200 mg/m2/day three times a day continuously in 28 day cycles. FLT3 mutated pts (either FT3-ITD or FLT3-TKD) with primary AML, therapy-related AML and AML following an antecedent hematological disorder were included. Pts were ≥18 years of age with ECOG PS of 0-2. Pts with CNS disease were excluded. Pts relapsing post-allogeneic stem cell transplant could be included if they were >30 days post-transplant. 38 pts enrolled in 2 parallel cohorts of which 34 were evaluable, 13 in cohort A (FLT3 TKI-naïve) and 21 in cohort B (progressed on prior FLT3 TKI).
Results
Median age was 61 (30 – 87). Patients had undergone a median of 3.5 prior therapies (range 1 – 8); 38% of pts had diploid and 23% complex cytogenetics. Among patients with available information, NPM1 mutation was identified in 62% (10/16) and DNMT3A in 57% (8/14). Median baseline marrow blast % was 58% (7 – 97%). Of cohort B patients, prior therapy was sorafenib in 57%, quizartinib in 14%, PLX3397 in 5% and midostaurin in 10%. 9% and 5% had received 2 and 3 FLT3 TKIs, respectively. 10 enrolled patients had progressed post-transplant (SCT) (3 allogeneic in cohort A; 6 allogeneic, 1 autologous in cohort B). Median duration of study therapy was 9 wks (range 5 – 24), 2 pts remain on study. Reasons for study cessation were disease progression in 66%, no response in 16%, toxicity in 6%, clinical deterioration due to other co-morbidities in 6%, 3% lost to follow up and 3% to receive SCT. At a median follow up of 14 weeks (wks), ORR was 47%: 12% achieved complete response with incomplete count recovery (CRi), 32% hematological improvement (Hi) (85% of them with >50% decrease in blast count) and 3% morphologic leukemia-free state (MLFS). 21% had progressive disease and 32% no response.
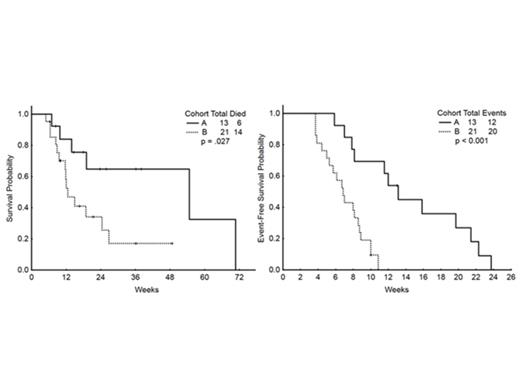
The median event-free survival (EFS) was 8 wks and overall survival (OS) was 19 wks for the whole cohort. The response by cohort was:
| . | FLT3 TKI naïve . | Prior FLT3 Therapy . |
|---|---|---|
| Response % | ||
| CRi | 23 | 5 |
| MLFS | 8 | 0 |
| Hi | 31 | 33 |
| NR | 31 | 33 |
| PD | 8 | 29 |
| Median OS | 55 wks | 13 wks (p=0.027) |
| Median EFS | 13 wks | 7 wks (p<0.001) |
| . | FLT3 TKI naïve . | Prior FLT3 Therapy . |
|---|---|---|
| Response % | ||
| CRi | 23 | 5 |
| MLFS | 8 | 0 |
| Hi | 31 | 33 |
| NR | 31 | 33 |
| PD | 8 | 29 |
| Median OS | 55 wks | 13 wks (p=0.027) |
| Median EFS | 13 wks | 7 wks (p<0.001) |
Pts achieving marrow response (CRi and MLFS) had superior EFS (median 22 wks vs 8 wks for non-responders, p=0.003), with a trend toward superior OS for the marrow responders (median 55 weeks versus 15 wks, p=0.166). 2 of the 4 pts with FLT3-TKD in cohort A responded (CRi, Hi). Among pts with both mutations, 1/1 pt in cohort A achieved CRi and 5/6 pts in cohort B achieved Hi.
EFS and OS were not influenced by complex cytogenetics, number of prior therapies or the presence of NPM1 or DNMT3A mutations. OS and EFS were, for patients with ITD (19 wks; 7 wks), D835 (6 wks; 8 wks) or both FLT3 mutations (12 wks; 9 wks), p=0.908; 0.391 respectively.
The main grade 3 toxicities were GI side effects (abdominal pain and nausea). There was no death attributed to treatment related toxicity.
Conclusions
Crenolanib is a well-tolerated FLT3 TKI with clinical efficacy in heavily pre-treated, relapsed/refractory FLT3 mutated AML patients. The superior results in FLT3 inhibitor-naïve patients suggests that on-target FLT3 inhibition is likely primarily responsible for drug efficacy. Combination therapy (e.g., with chemotherapy or hypomethylating agents) could potentially provide additive efficacy in both treatment-naïve and relapsed/refractory pts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal