Key Points
Coordinated BCR-ABL1 kinase-dependent and -independent mechanisms convert p27 from a nuclear tumor suppressor to a cytoplasmic oncogene.
Oncogenic functions of p27 that persist despite effective BCR-ABL1 inhibition may contribute to resistance to tyrosine kinase inhibitors.
Abstract
Recent studies have revealed that p27, a nuclear cyclin-dependent kinase (Cdk) inhibitor and tumor suppressor, can acquire oncogenic activities upon mislocalization to the cytoplasm. To understand how these antagonistic activities influence oncogenesis, we dissected the nuclear and cytoplasmic functions of p27 in chronic myeloid leukemia (CML), a well-characterized malignancy caused by the BCR-ABL1 tyrosine kinase. p27 is predominantly cytoplasmic in CML and nuclear in normal cells. BCR-ABL1 regulates nuclear and cytoplasmic p27 abundance by kinase-dependent and -independent mechanisms, respectively. p27 knockdown in CML cell lines with predominantly cytoplasmic p27 induces apoptosis, consistent with a leukemogenic role of cytoplasmic p27. Accordingly, a p27 mutant (p27CK−) devoid of Cdk inhibitory nuclear functions enhances leukemogenesis in a murine CML model compared with complete absence of p27. In contrast, p27 mutations that enhance its stability (p27T187A) or nuclear retention (p27S10A) attenuate leukemogenesis over wild-type p27, validating the tumor-suppressor function of nuclear p27 in CML. We conclude that BCR-ABL1 kinase-dependent and -independent mechanisms convert p27 from a nuclear tumor suppressor to a cytoplasmic oncogene. These findings suggest that cytoplasmic mislocalization of p27 despite BCR-ABL1 inhibition by tyrosine kinase inhibitors may contribute to drug resistance, and effective therapeutic strategies to stabilize nuclear p27 must also prevent cytoplasmic mislocalization.
Introduction
p27, an inhibitor of cyclin-dependent kinases (Cdks), is a key regulator of cell-cycle progression in mammalian cells.1,2 p27 abundance is tightly controlled throughout the cell cycle by a complex set of mechanisms.2 Although p27 is considered a tumor suppressor, mutations in human cancer are exceedingly rare and tumors developing in p27−/+ mice do not exhibit loss of the remaining p27 allele.3-5 These unusual properties suggest that p27 is a haploinsufficient tumor suppressor.5 Recently, previously unappreciated oncogenic activity of cytoplasmic p27 has been uncovered using knock-in mice expressing a p27 mutant (p27CK−) lacking nuclear cyclin/Cdk inhibitory functions.6,7 These mice have a higher rate of spontaneous tumors than p27−/− mice, suggesting cytoplasmic p27 actively promotes oncogenesis. Consistent with this, cytoplasmic p27 increased oncogenicity in melanoma8 and breast cancer9 xenograft models, and a low nuclear:cytoplasmic ratio of p27 is an adverse prognostic marker in solid tumors.10
We studied the antagonistic nuclear and cytoplasmic functions of p27 in chronic myeloid leukemia (CML), a well-characterized myeloproliferative neoplasm caused by the BCR-ABL1 tyrosine kinase.11,12 Unlike normal cells, CML CD34+ cells enter the S phase of the cell cycle in the absence of cytokines.13 Several studies demonstrated that BCR-ABL1 compromises p27 function by various mechanisms.14,15 For example, BCR-ABL1 activates phosphatidylinositol 3-kinase (PI3K)/AKT, which inhibits p27 transcription through phosphorylation and inhibition of Forkhead/FoxO transcription factors.16 Additionally, PI3K induces expression of SKP2, the F-box protein of the SCFSKP2 ubiquitin E3 ligase complex, promoting degradation of nuclear p27 after phosphorylation of threonine-187 by cyclin E/Cdk2.17,18 The latter is enhanced by BCR-ABL1–induced phosphorylation of p27 on tyrosine-88, which releases cyclin E/Cdk2 from p27 inhibition, increasing Cdk2 activity.19 Lastly, cytoplasmic relocalization of p27 in CML cells may shelter Cdks from p27 inhibition, facilitating cell-cycle progression.20,21
We used biochemical assays and murine models to dissect the opposing roles of p27 in the nuclear and cytoplasmic compartments of CML cells. We show that BCR-ABL1 coordinates the conversion of p27 from a nuclear tumor suppressor into a cytoplasmic oncogene, using kinase-dependent mechanisms to reduce nuclear p27 levels and kinase-independent mechanisms to promote cytoplasmic mislocalization. Our studies suggest that effective therapeutic strategies to stabilize nuclear p27 must simultaneously prevent abnormal cytoplasmic localization.
Materials and methods
Patient samples and cell lines
Samples from CML patients treated at Oregon Health & Science University (OHSU) or at The MD Anderson Cancer Center were obtained following informed consent. Studies using human cells were approved by the institutional internal review boards. Marrow from normal donors was purchased from a commercial vendor (Lonza). Research was conducted in accordance with the Declaration of Helsinki. Cell lines were maintained as described in supplemental Methods (see supplemental Data available at the Blood Web site).18
Immunoblot analysis
Whole-cell lysates and nuclear-cytoplasmic fractions were prepared as described in supplemental Methods.18
MEFs and retroviral infection
Lentiviral infection
Short-hairpin RNA (shRNA) sequences in the pLKO1 vector targeting human p27 (Open Biosystems) were subcloned into the FUGW vector.23 Lentivirus generation and stable transfection were performed as described in supplemental Methods.
Mice
C57BL/6 p27+/− mice5,24 were purchased (The Jackson Laboratory). Breeding pairs for p27T187A in the C57BL/6 background25 and p27+/−, p27S10A, and p27CK− knock-in mice in the 129S4 background6,22 were received from Fred Hutchison Cancer Center and maintained in the OHSU animal care facility.5,6,22,24,25
In vitro transformation and BM transplantation assays
Immunofluorescence, immunohistochemical, and RNA-Seq analyses
Immunofluorescence, immunohistochemical, and RNA-Seq analyses were performed as described in supplemental Methods.
Results
p27 is predominantly cytoplasmic in CML cells
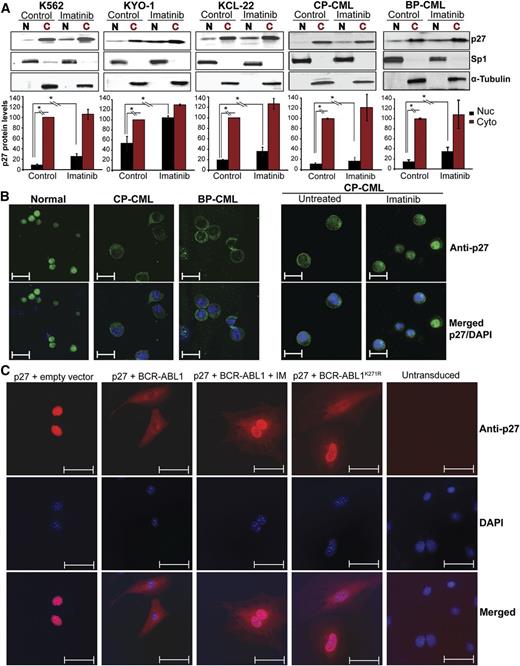
Immunoblot analysis of total p27 expression and subcellular localization in BCR-ABL1–positive cell lines, CD34+ cells from newly diagnosed chronic phase (CP; N = 8) and blastic phase (BP; N = 3) CML patients, and normal CD34+ controls demonstrated predominantly cytoplasmic localization in CML cell lines and in CML CD34+ cells from 10 of 11 patients, but mostly nuclear localization in normal controls (Figure 1A-B; supplemental Figure 1A-B). Total p27 expression was similar in CML and normal CD34+ cells (supplemental Figure 2A,B,D).
Effect of BCR-ABL1 kinase inhibition and kinase-inactive BCR-ABL1 on subcellular localization of p27 in CML cells. (A) CML cell lines (K562, KYO-1, and KCL-22) and CD34+ cells from CML patients (N = 11) in CP (N = 8) or BP (N = 3) were treated with imatinib for 16 hours. Cell lines were serum starved in 1% serum and primary samples were cultured in cytokine-free 20% BIT media before performing the experiments. Cytoplasmic (C) and nuclear (N) proteins were fractionated and subjected to immunoblot analysis, using Sp1 and α-tubulin distribution to monitor the purity of the nuclear and cytoplasmic fractions, respectively. Representative immunoblots are shown. Densitometry was performed by normalizing nuclear and cytoplasmic p27 protein levels to levels of Sp1 and α-tubulin, respectively. Expression of untreated cytoplasmic p27 levels was set to 100%. Values represent mean ± SD from 3 independent experiments. *P < .050. (B) Subcellular localization of p27 was analyzed by immunofluorescence (IF) microscopy in CD34+ cells from BM of normal donors or CML patients. Left panel, cells were freshly isolated; right panel, cells were treated with imatinib in cytokine-free 20% BIT media. Representative experiments are shown. Scale bars represent 10 μm. (C) p27−/− MEFs were stably cotransduced with RFP-p27 (wild type) and GFP retroviral vectors containing native BCR-ABL1, the kinase-inactive BCR-ABL1K271R mutant, or empty vector controls. Cells engineered to express native BCR-ABL1 were also treated with 2.5 μM imatinib for 16 hours. Double-positive (GFP+/RFP+) cells were sorted by FACS and the subcellular localization of p27 was visualized by IF using an Alexa Fluor-647–conjugated secondary antibody. Coexpression of GFP and RFP was verified under the microscope. DAPI was used for nuclear staining. Scale bars represent 20 μm. Quantification of nuclear/cytoplasmic p27 signal intensity is shown in supplemental Figure 3. BIT, bovine serum albumin, insulin, and transferrin; DAPI, 4,6 diamidino-2-phenylindole; GFP, green fluorescent protein; RFP, red fluorescent protein.
Effect of BCR-ABL1 kinase inhibition and kinase-inactive BCR-ABL1 on subcellular localization of p27 in CML cells. (A) CML cell lines (K562, KYO-1, and KCL-22) and CD34+ cells from CML patients (N = 11) in CP (N = 8) or BP (N = 3) were treated with imatinib for 16 hours. Cell lines were serum starved in 1% serum and primary samples were cultured in cytokine-free 20% BIT media before performing the experiments. Cytoplasmic (C) and nuclear (N) proteins were fractionated and subjected to immunoblot analysis, using Sp1 and α-tubulin distribution to monitor the purity of the nuclear and cytoplasmic fractions, respectively. Representative immunoblots are shown. Densitometry was performed by normalizing nuclear and cytoplasmic p27 protein levels to levels of Sp1 and α-tubulin, respectively. Expression of untreated cytoplasmic p27 levels was set to 100%. Values represent mean ± SD from 3 independent experiments. *P < .050. (B) Subcellular localization of p27 was analyzed by immunofluorescence (IF) microscopy in CD34+ cells from BM of normal donors or CML patients. Left panel, cells were freshly isolated; right panel, cells were treated with imatinib in cytokine-free 20% BIT media. Representative experiments are shown. Scale bars represent 10 μm. (C) p27−/− MEFs were stably cotransduced with RFP-p27 (wild type) and GFP retroviral vectors containing native BCR-ABL1, the kinase-inactive BCR-ABL1K271R mutant, or empty vector controls. Cells engineered to express native BCR-ABL1 were also treated with 2.5 μM imatinib for 16 hours. Double-positive (GFP+/RFP+) cells were sorted by FACS and the subcellular localization of p27 was visualized by IF using an Alexa Fluor-647–conjugated secondary antibody. Coexpression of GFP and RFP was verified under the microscope. DAPI was used for nuclear staining. Scale bars represent 20 μm. Quantification of nuclear/cytoplasmic p27 signal intensity is shown in supplemental Figure 3. BIT, bovine serum albumin, insulin, and transferrin; DAPI, 4,6 diamidino-2-phenylindole; GFP, green fluorescent protein; RFP, red fluorescent protein.
Cytoplasmic localization of p27 is directed by BCR-ABL1 in a kinase-independent manner
To determine whether cytoplasmic localization of p27 is regulated by BCR-ABL1 kinase activity, we treated CML cells with imatinib (16 hours). We observed a 1.5-fold increase in the G0/G1 fraction, which is correlated with increased p27 and decreased SKP2 expression (supplemental Figure 2C-D). Analysis of cytoplasmic and nuclear fractions revealed increased nuclear p27 upon imatinib treatment (Figure 1A-B; supplemental Figure 1A-B), whereas cytoplasmic p27 expression was unchanged, suggesting that BCR-ABL1 regulates nuclear and cytoplasmic p27 in a kinase-dependent and kinase-independent manner, respectively. To determine whether BCR-ABL1 kinase activity is required to shift p27 from the nucleus to the cytoplasm, we coexpressed FLAG-tagged wild-type p27 (RFP+) with kinase-active BCR-ABL1 or kinase-inactive BCR-ABL1K271R (GFP+) in p27−/− MEFs. p27 localization was analyzed by IF in doubly-transduced cells. Without ectopic BCR-ABL1, p27 was mostly nuclear and the nuclear/cytoplasmic ratio was high. Upon expression of kinase-active BCR-ABL1, nuclear p27 decreased, whereas cytoplasmic p27 became dominant (Figure 1C; supplemental Figure 3). Imatinib treatment of BCR-ABL1–expressing MEFs led to nuclear p27 accumulation without affecting cytoplasmic p27 levels; expression of kinase-inactive BCR-ABL1K271R produced identical results. Experiments using 32Dcl3 murine myeloid progenitor cells yielded comparable data (supplemental Figure 4). We conclude that nuclear p27 is regulated by BCR-ABL1 in a kinase-dependent manner, whereas cytoplasmic p27 is regulated in a kinase-independent manner. To probe which motifs in BCR-ABL1 are required to localize p27 to the cytoplasm, we expressed a series of BCR-ABL1 mutants in 32Dcl3 cells and/or in p27−/− MEFs reconstituted with p27 (supplemental Table 1, supplemental Figure 5A-D). All tested BCR-ABL1 mutants, including a triple mutant carrying a Y177F mutation together with deletions of the Src homology 2 domain and the proline-rich region,27 retained the ability to shift p27 to the cytoplasm. Furthermore, treatment of K562 cells with several signaling pathway inhibitors failed to influence the nuclear/cytoplasmic distribution of p27 (supplemental Table 2, supplemental Figure 6). Previous studies suggested that in some CML cell lines the small JAB1 complex promotes cytoplasmic localization and degradation of p27.28 To test whether JAB1 levels may correlate with cytoplasmic p27 localization, we analyzed JAB1 expression by IF microscopy in CML cells expressing predominantly cytoplasmic (K562 and KYO-1) or nuclear p27 (Mo7ep210). K562 and KYO-1 cells expressed higher levels of JAB1 compared with Mo7ep210 cells, suggesting that JAB1 may be involved in cytoplasmic p27 localization (supplemental Figure 7). Furthermore, consistent with the lack of an imatinib effect on cytoplasmic p27 levels, JAB1 expression was unchanged upon imatinib inhibition of BCR-ABL1.
Cytoplasmic p27 promotes survival of CML cells
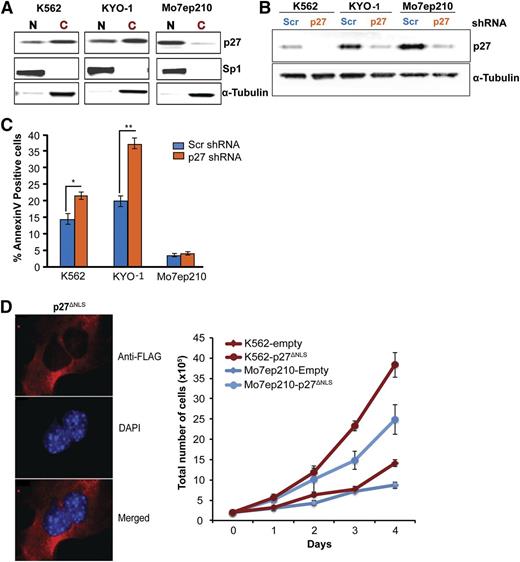
To interrogate cytoplasmic p27 function, we transduced CML cells expressing predominantly cytoplasmic p27 (K562, KYO-1) (Figure 2A) with a lentiviral vector expressing a shRNA targeting p27. Mo7ep210 cells expressing mostly nuclear p27 served as controls. Infection efficiency was >90% in all lines (supplemental Figure 8), and total p27 was reduced to low levels (Figure 2B). p27 knockdown in CML cells expressing predominantly cytoplasmic p27 increased apoptosis, whereas Mo7ep210 cells were unaffected, suggesting cytoplasmic p27 promotes CML cell survival (Figure 2C), but had no effect on adhesion to fibronectin (data not shown). To further validate the role of cytoplasmic p27, we ectopically expressed a p27 mutant lacking the nuclear localization signal (p27ΔNLS) in K562 and Mo7ep210 cell lines and found that this exclusively cytoplasmic p27 mutant promoted proliferation in both cell lines (Figure 2D). As a recent study implicated RHO A and SAPK/c-Jun N-terminal kinase (JNK) as a downstream effector of cytoplasmic p27,29 we assayed phosphorylation of cofilin, a regulator of actin dynamics and downstream effector of RHO A. Downregulation of p27 in K562 cells did not affect cofilin phosphorylation (data not shown).
Cytoplasmic p27 promotes survival of CML cells. (A) p27 immunoblotting was performed on the nuclear and cytoplasmic fractions of exponentially growing CML cell lines. K562 and KYO-1 cells show predominantly cytoplasmic p27, whereas Mo7ep210 cells show predominantly nuclear p27. (B) K562, KYO-1, and Mo7ep210 cells were infected with p27 or scrambled shRNA lentivirus and total p27 expression was determined by immunoblot analysis. (C) Cells infected with p27 or scrambled shRNA were analyzed for apoptosis using Annexin V staining. *P < .050; **P < .010. (D) Effect of cytoplasmic-only p27 (p27ΔNLS) on cell growth of CML cells. p27−/− MEFs were transduced with Flag-p27ΔNLS and localization was validated by IF analysis using anti-Flag antibody (M2; Sigma-Aldrich). DAPI was used for nuclear staining. K562 and Mo7ep210 cells were transduced with p27ΔNLS or empty vector control and the effect on cell growth was measured over time.
Cytoplasmic p27 promotes survival of CML cells. (A) p27 immunoblotting was performed on the nuclear and cytoplasmic fractions of exponentially growing CML cell lines. K562 and KYO-1 cells show predominantly cytoplasmic p27, whereas Mo7ep210 cells show predominantly nuclear p27. (B) K562, KYO-1, and Mo7ep210 cells were infected with p27 or scrambled shRNA lentivirus and total p27 expression was determined by immunoblot analysis. (C) Cells infected with p27 or scrambled shRNA were analyzed for apoptosis using Annexin V staining. *P < .050; **P < .010. (D) Effect of cytoplasmic-only p27 (p27ΔNLS) on cell growth of CML cells. p27−/− MEFs were transduced with Flag-p27ΔNLS and localization was validated by IF analysis using anti-Flag antibody (M2; Sigma-Aldrich). DAPI was used for nuclear staining. K562 and Mo7ep210 cells were transduced with p27ΔNLS or empty vector control and the effect on cell growth was measured over time.
The net function of p27 in BCR-ABL1–induced leukemia is tumor-suppressive
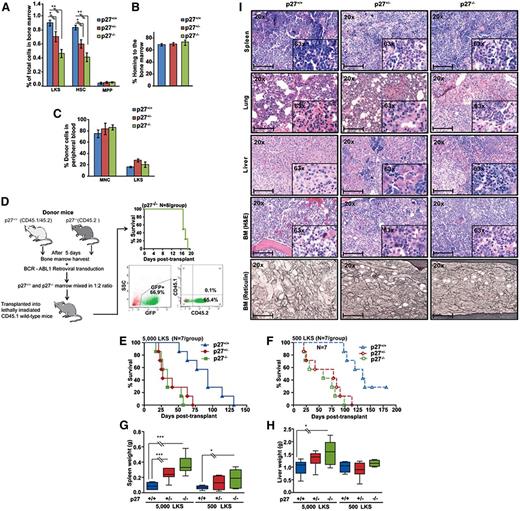
We next studied the function of p27 for BCR-ABL1 leukemogenesis in vivo, taking advantage of mouse strains with specific p27 mutations (supplemental Table 3). We used p27−/− mice to determine the net effect of p27 on leukemogenesis.24,30,31 To control for potential bias due to differences in progenitor cells and function, we subjected BM from p27+/+ and p27−/− mice to detailed fluorescence-activated cell sorter (FACS) analysis. The proportion of Lin−/c-Kit+/Sca1+ (LKS) and CD34− LKS cells was reduced by 50% in p27−/− and 25% in p27+/− compared with p27+/+ mice, whereas multipotent progenitor cells (CD34+ LKS) were identical (Figure 3A; supplemental Figure 9A). After normalization for total numbers of BM cells harvested from long bones, stem cell numbers were similar in p27−/− mice compared with p27+/+ mice, whereas multipotent progenitors were increased, consistent with previous observations (supplemental Figure 9B).32 Homing (Figure 3B) and long-term reconstituting capacity (Figure 3C) of p27−/− and p27+/+ LKS cells were identical, indicating that p27−/− LKS cells are proportionately reduced but functionally normal. To delineate the role of p27 in BCR-ABL1–mediated leukemic transformation, we performed serial replating assays. BCR-ABL1–transduced p27−/− cells had significantly increased clonogenic potential as compared with BCR-ABL1–transduced p27+/+ cells (supplemental Figure 10). Next, we determined the role of p27 in in vivo leukemogenesis in a competitive BM transplantation experiment, adjusting for the 50% proportionate reduction of BM LKS cells in p27−/− mice. Lethally irradiated p27+/+ congenic recipients (CD45.1) received 1.4 × 105 per mouse BCR-ABL1–transduced BM cells from p27−/− CD45.2 donors plus 0.7 × 105 BCR-ABL1–transduced BM cells from p27+/+ CD45.2/CD45.1 mice. Within 19 days of transplantation all mice died of leukemia originating from p27−/− BM cells, as shown by absence of CD45.1 expression (Figure 3D). Because CML in this model can arise from transient expansion of progenitor rather than stem cells,33 it remained possible that the effect of p27 was restricted to progenitor cells. We therefore sorted GFP+ LKS cells from BCR-ABL1–transduced p27−/−, p27+/−, and p27+/+ CD45.2 BM (supplemental Figure 11A) and injected equal numbers (5000 or 500 LKS cells per mouse) into lethally irradiated CD45.1 congenic mice (Figure 3E-F). Recipients of 5000 p27−/− GFP+ LKS cells died of CML-like disease at a median of 34 days (range, 17-57), whereas recipients of 5000 p27+/+ cells survived for a median of 91 days (range, 51-131) (P < .001). Median survival of mice transplanted with p27+/− GFP+ LKS cells was 27 days (range, 21-71), significantly different from p27+/+ (P < .001) but not p27−/− mice (P = .560) (Figure 3E). Results with 500 cells per mouse were analogous (Figure 3F). Recipients of 5000 p27−/− LKS cells had larger spleens and livers (Figure 3G-H), higher white blood cell count (WBC) and higher platelet counts compared with recipients of p27+/+ LKS cells (supplemental Table 4). Histopathology revealed CML-like disease with hepatosplenic extramedullary hematopoiesis, hypercellular BM with reticulin fibrosis, and focal pulmonary hemorrhage. Findings were consistently more pronounced in recipients of BCR-ABL1–transduced p27−/− and p27+/− LKS vs recipients of p27+/+ LKS cells (Figure 3I). Immunophenotyping of BM and spleen cells revealed that the majority of GFP+ cells expressed granulocytic markers (Gr1 and CD11b), whereas fewer cells expressed T- (CD3) or B-cell (CD19) antigens. LKS cells represented a minority of cells, with no significant differences between groups (supplemental Figure 11B). Consistent with BCR-ABL1–mediated downregulation of nuclear p27, immunohistochemistry (IHC) analysis of BM from leukemic mice and IF analysis of BCR-ABL1–transduced MEFs showed a significantly reduced nuclear/cytoplasmic p27 ratio as compared with empty vector (EV) control (supplemental Figure 12A-B). Altogether, these results support a net tumor-suppressive effect of p27 in this CML model.
The net function of p27 in BCR-ABL1–mediated leukemogenesis is tumor-suppressive. (A) BM harvested from 5-fluorouracil–treated p27+/+, p27+/−, and p27−/− mice (N = 3 per group) was analyzed by FACS to determine the proportions of Lin−/c-Kit+/Sca1+ (LKS) cells. The LKS population was gated for CD34 expression to distinguish between progenitor cells (LKS CD34+ for MPP) and the stem cell–enriched fraction (LKS CD34− for HSC). (B) Homing capacity was assessed in recipients of BM LKS cells from p27+/+, p27+/−, and p27−/− mice (N = 3 per group) using CFSE labeling. (C) Long-term engraftment was assessed in CD45.1+ recipients of CD45.2+ BM cells from p27+/+, p27+/− and p27−/− mice (N = 3 per group) after injecting all mice with MNCs (3 × 105 cells per mouse) and LKS cells (3000 cells per mouse). Starting 4 weeks after transplantation, the contribution of CD45.2+ cells in the peripheral blood was measured by weekly FACS. The data shown represent engraftment after 6 weeks. (D) BCR-ABL1–expressing p27+/+ and p27−/− BM cells were subjected to an in vivo competition experiment. To adjust for the 50% reduced LKS population in p27−/− mice, twice the number of BCR-ABL1–transduced BM cells from p27−/− CD45.2 mice (1.4 × 105 cells per mouse) mixed with BCR-ABL1–transduced BM cells from p27+/+ CD45.2/CD45.1 mice (0.7 × 105 cells per mouse) were injected into lethally irradiated wild-type congenic recipient mice (CD45.1). At the time of autopsy, BM cells were analyzed by FACS for the presence of GFP+ p27−/− CD45.2 or p27+/+ CD45.1 cells. (E-F) p27+/+ mice were transplanted with BCR-ABL1–transduced GFP+ BM LKS cells (5000 or 500 per mouse) from p27+/+, p27+/− and p27−/− mice and compared for survival using Kaplan-Meier statistics. (G-H) Spleen and liver weights of mice transplanted with BCR-ABL1–transduced LKS cells were compared according to genotype. (I) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced LKS cells. Scale bars represent 100 μm *P < .050; **P < .010; **P < .001. CFSE, carboxyfluorescein succinimidyl ester; HSC, hematopoietic stem cells; MNC, mononuclear cell; MPP, multipotent progenitor.
The net function of p27 in BCR-ABL1–mediated leukemogenesis is tumor-suppressive. (A) BM harvested from 5-fluorouracil–treated p27+/+, p27+/−, and p27−/− mice (N = 3 per group) was analyzed by FACS to determine the proportions of Lin−/c-Kit+/Sca1+ (LKS) cells. The LKS population was gated for CD34 expression to distinguish between progenitor cells (LKS CD34+ for MPP) and the stem cell–enriched fraction (LKS CD34− for HSC). (B) Homing capacity was assessed in recipients of BM LKS cells from p27+/+, p27+/−, and p27−/− mice (N = 3 per group) using CFSE labeling. (C) Long-term engraftment was assessed in CD45.1+ recipients of CD45.2+ BM cells from p27+/+, p27+/− and p27−/− mice (N = 3 per group) after injecting all mice with MNCs (3 × 105 cells per mouse) and LKS cells (3000 cells per mouse). Starting 4 weeks after transplantation, the contribution of CD45.2+ cells in the peripheral blood was measured by weekly FACS. The data shown represent engraftment after 6 weeks. (D) BCR-ABL1–expressing p27+/+ and p27−/− BM cells were subjected to an in vivo competition experiment. To adjust for the 50% reduced LKS population in p27−/− mice, twice the number of BCR-ABL1–transduced BM cells from p27−/− CD45.2 mice (1.4 × 105 cells per mouse) mixed with BCR-ABL1–transduced BM cells from p27+/+ CD45.2/CD45.1 mice (0.7 × 105 cells per mouse) were injected into lethally irradiated wild-type congenic recipient mice (CD45.1). At the time of autopsy, BM cells were analyzed by FACS for the presence of GFP+ p27−/− CD45.2 or p27+/+ CD45.1 cells. (E-F) p27+/+ mice were transplanted with BCR-ABL1–transduced GFP+ BM LKS cells (5000 or 500 per mouse) from p27+/+, p27+/− and p27−/− mice and compared for survival using Kaplan-Meier statistics. (G-H) Spleen and liver weights of mice transplanted with BCR-ABL1–transduced LKS cells were compared according to genotype. (I) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced LKS cells. Scale bars represent 100 μm *P < .050; **P < .010; **P < .001. CFSE, carboxyfluorescein succinimidyl ester; HSC, hematopoietic stem cells; MNC, mononuclear cell; MPP, multipotent progenitor.
Stabilization of nuclear p27 prolongs survival of mice with BCR-ABL1–induced leukemia
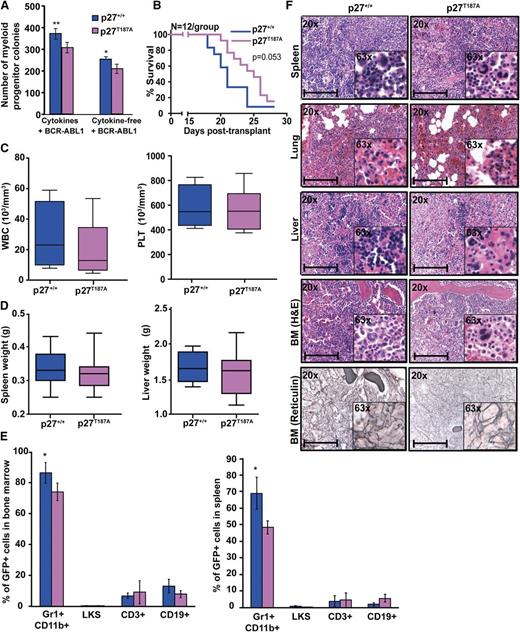
We next tested whether stabilization of nuclear p27 contributes to its net tumor-suppressive effect using mice expressing a mutant form of p27 (p27T187A) that is resistant to degradation by SCFSKP2 during G1 and S phase.25,34 Because BCR-ABL1 regulates SKP2-mediated degradation of phospho-p27T187 in the nucleus,18 we hypothesized that p27T187A will counteract BCR-ABL1 effects on the nuclear p27 pool. Immunofluorescence analysis of p27T187A MEFs revealed that the p27 nuclear/cytoplasmic ratio was similar in EV-transduced p27+/+ and p27T187A cells. In contrast, BCR-ABL1–transduced p27T187A cells had a significantly higher nuclear/cytoplasmic p27 ratio compared with BCR-ABL1–transduced p27+/+ cells (supplemental Figure 12A). No significant differences in the proportion and absolute number of LKS cells were observed between p27T187A and p27+/+ mice (supplemental Figure 13A-B). However, BCR-ABL1–mediated myeloid colony formation as well as serial replating potential was reduced in p27T187A cells (Figure 4A; supplemental Figure 10). We then transplanted equal numbers of BCR-ABL1–transduced BM cells (2 × 106 cells/mouse with 1.9% GFP+ cells) from p27+/+ and p27T187A mice into lethally irradiated p27+/+ recipients. Both p27+/+ and p27T187A mice developed CML-like disease, but recipients of p27T187A marrow survived for a median of 25 days (range, 20-27) compared with 21 days (range, 18-24) for recipients of p27+/+ marrow (Figure 4B; P = .053). Mice transplanted with p27T187A marrow and p27+/+ marrow showed comparable WBC and platelet counts (Figure 4C). However, p27T187A leukemic mice tended to have less splenomegaly (Figure 4D), showed a significant reduction in myeloid precursors in BM and spleen (Figure 4E) and had slightly reduced myeloid infiltrates in spleen, liver, and lungs, reduced BM cellularity, and less reticulin fibrosis (Figure 4F). IHC analysis on BM showed an increased nuclear/cytoplasmic p27 ratio in BCR-ABL1–transformed p27T187A as compared with BCR-ABL1–transformed p27+/+cells (supplemental Figure 12B). Overall, our results show that stabilization of nuclear p27 attenuates disease progression, in accordance with a tumor-suppressor role of nuclear p27.
Stabilization of p27 in the nucleus attenuates BCR-ABL1–induced leukemia. (A) BM cells from p27+/+ and p27T187A mice were transduced with BCR-ABL1 retrovirus, sorted by FACS for GFP+/Lin− cells, and plated in methylcellulose in the presence or absence of cytokines. Myeloid colony formation of BCR-ABL1–transduced cells was assessed after 8 days. (B) p27+/+ mice were transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27T187A mice and survival was analyzed using Kaplan-Meier statistics. (C) WBC and PLT counts and (D) spleen and liver weights of leukemic mice were compared according to genotype. (E) Lineage distributions of GFP+ cells in the bone marrow and spleen of leukemic mice: granulocytes (Gr1+CD11b+), B cells (CD19+), T cells (CD3+), and LKS cells (Lin−c-Kit+Sca1+). Error bars represent standard deviation. (F) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27T187A mice. Scale bars represent 100 μm. In the above experiments, *P < .050, **P < .010. PLT, platelet.
Stabilization of p27 in the nucleus attenuates BCR-ABL1–induced leukemia. (A) BM cells from p27+/+ and p27T187A mice were transduced with BCR-ABL1 retrovirus, sorted by FACS for GFP+/Lin− cells, and plated in methylcellulose in the presence or absence of cytokines. Myeloid colony formation of BCR-ABL1–transduced cells was assessed after 8 days. (B) p27+/+ mice were transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27T187A mice and survival was analyzed using Kaplan-Meier statistics. (C) WBC and PLT counts and (D) spleen and liver weights of leukemic mice were compared according to genotype. (E) Lineage distributions of GFP+ cells in the bone marrow and spleen of leukemic mice: granulocytes (Gr1+CD11b+), B cells (CD19+), T cells (CD3+), and LKS cells (Lin−c-Kit+Sca1+). Error bars represent standard deviation. (F) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27T187A mice. Scale bars represent 100 μm. In the above experiments, *P < .050, **P < .010. PLT, platelet.
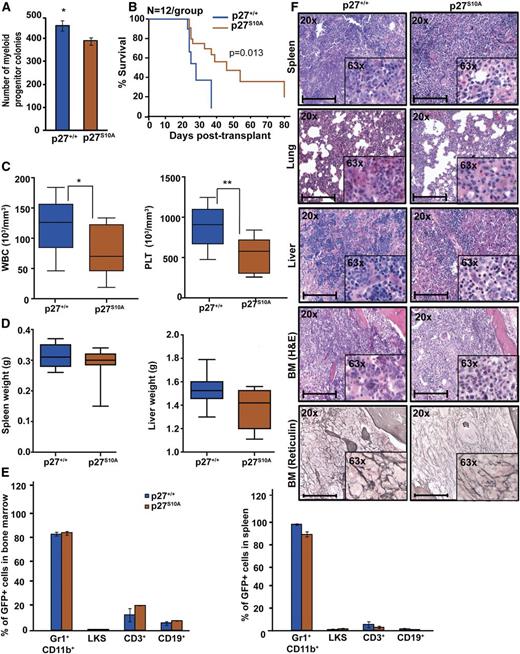
Nuclear retention of p27 prolongs survival of mice with BCR-ABL1–induced leukemia
To further dissect the role of nuclear and cytoplasmic p27 for BCR-ABL1–mediated leukemogenesis, we used p27S10A knock-in mice in which the serine phosphorylation site, implicated in nuclear export and protein stability, is replaced with alanine, leading to reduced cytoplasmic and total p27.22,35,36 To confirm the effect of the p27S10A mutation on subcellular localization, we performed IF on EV and BCR-ABL1–transduced p27S10A MEFs. The nuclear/cytoplasmic p27 ratio in p27S10A EV-transduced MEFs was slightly lower than in p27+/+ cells, suggesting that the S10A mutation reduces protein stability. We found no significant difference in the nuclear/cytoplasmic ratio between BCR-ABL1–expressing p27+/+ vs p27S10A cells. This suggests that BCR-ABL1 may activate 1 or more of the kinases reported to phosphorylate p27 on S10, such as human kinase interacting stathmin.37 However, BCR-ABL1–expressing p27S10A cells exhibit a significantly increased nuclear/cytoplasmic p27 ratio as compared with p27+/+ cells, demonstrating that the increased nuclear retention of p27 overrides the S10A-related decrease in stability (supplemental Figure 12A). BCR-ABL1–mediated myeloid colony formation with BCR-ABL1–transduced p27S10A cells (Figure 5A) as well as serial replating assays showed significantly reduced clonogenic potential as compared with BCR-ABL1–transduced p27+/+ cells (supplemental Figure 10). Immunophenotyping of p27S10A BM showed increased LKS cells compared with p27+/+ marrow (supplemental Figure 14A-B), whereas engraftment was similar (supplemental Figure 14C). Despite the increased LKS numbers, recipients of BCR-ABL1–transduced p27S10A BM cells exhibited prolonged survival (median, 32 days; range, 23-79) as compared with recipients of equal numbers of BCR-ABL1–transduced p27+/+ cells (median, 23 days; range, 21-38; P = .013; Figure 5B). WBC and platelet counts in p27S10A leukemic mice were also lower (Figure 5C), and there was a slight but not statistically significant reduction in spleen and liver weights (Figure 5D). Although the percentages of myeloid precursors, LKS, T, or B cells in BM and spleen were similar (Figure 5E), histopathology revealed reduced leukemic infiltrates in spleen, liver, and lungs and reduced BM fibrosis in p27S10A leukemic mice compared with p27+/+ mice (Figure 5F). Consistent with p27S10A MEFs (supplemental Figure 12A), IHC analysis of BM showed a reduced nuclear/cytoplasmic p27 ratio in EV-transduced p27S10A cells and increased nuclear/cytoplasmic p27 ratio in BCR-ABL1–transduced p27S10A cells as compared with p27+/+ controls (supplemental Figure 12B). Overall, these results suggest that increased retention of p27 in the nucleus attenuates BCR-ABL1–driven leukemia.
Retention of p27 in the nucleus attenuates BCR-ABL1–induced leukemia. (A) BM cells from p27+/+ and p27S10A mice were transduced with BCR-ABL1 retrovirus, sorted by FACS for GFP+/Lin– cells, and plated in methylcellulose in absence of cytokines. Myeloid colony formation of BCR-ABL1–transduced cells was assessed after 8 days. (B) Kaplan-Meier survival analysis of p27+/+ mice transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27S10A mice. (C) WBC and PLT counts and (D) spleen and liver weights of leukemic mice were compared according to genotype. (E) Lineage distributions of GFP+ cells in the BM and spleen of leukemic mice: granulocytes (Gr1+CD11b+), B cells (CD19+), T cells (CD3+), and LKS cells (Lin−c-Kit+Sca1+). Error bars represent standard deviation. (F) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27S10A mice. Scale bars represent 100 μm. In above experiments, *P < .050, **P < .010.
Retention of p27 in the nucleus attenuates BCR-ABL1–induced leukemia. (A) BM cells from p27+/+ and p27S10A mice were transduced with BCR-ABL1 retrovirus, sorted by FACS for GFP+/Lin– cells, and plated in methylcellulose in absence of cytokines. Myeloid colony formation of BCR-ABL1–transduced cells was assessed after 8 days. (B) Kaplan-Meier survival analysis of p27+/+ mice transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27S10A mice. (C) WBC and PLT counts and (D) spleen and liver weights of leukemic mice were compared according to genotype. (E) Lineage distributions of GFP+ cells in the BM and spleen of leukemic mice: granulocytes (Gr1+CD11b+), B cells (CD19+), T cells (CD3+), and LKS cells (Lin−c-Kit+Sca1+). Error bars represent standard deviation. (F) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced BM cells from p27+/+ and p27S10A mice. Scale bars represent 100 μm. In above experiments, *P < .050, **P < .010.
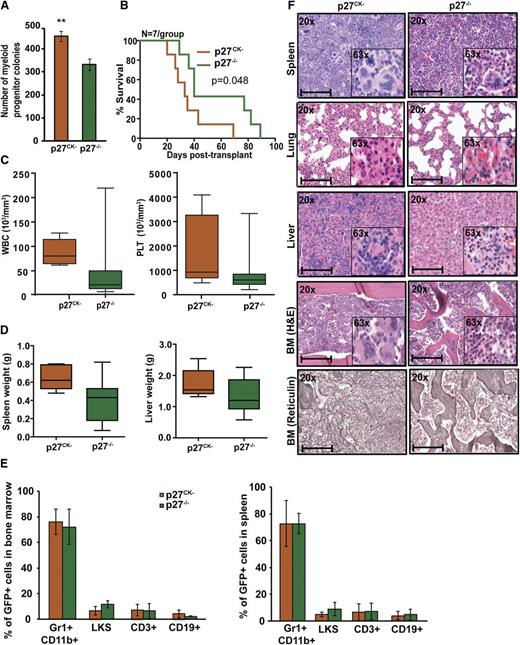
Cytoplasmic p27 promotes BCR-ABL1–induced leukemogenesis
To address the role of cytoplasmic p27 for leukemogenesis, we used p27CK− knock-in mice. These mice lack the nuclear Cdk inhibitory p27 activity due to mutation of cyclin and Cdk binding sequences, but retain cytoplasmic p27 functions.6 BM immunophenotyping demonstrated similar proportions of LKS cells in p27CK− and p27−/− mice (supplemental Figure 15A-B). BCR-ABL1–induced myeloid colony formation by p27CK− marrow was significantly increased compared with p27−/− marrow (P = .003) (Figure 6A). Similarly, in serial replating assays, the clonogenic capacity of BCR-ABL1–transduced p27CK− BM cells was significantly increased as compared with BCR-ABL1–transduced p27−/− cells (supplemental Figure 10). Upon transplantation of 5 × 105 cells per mouse (11% GFP+) recipients of p27CK− cells showed significantly reduced survival (median, 33 days; range, 20-69) compared with recipients of p27−/− cells (median, 40 days; range, 29-89; P = .048) (Figure 6B). WBC and platelet counts were increased but the difference was not statistically significant (Figure 6C); autopsy also revealed a 1.5-fold increase in spleen weight (P = .050) and slightly increased liver weight (Figure 6D). Although the proportions of myeloid precursors, LKS, T, or B cells in BM and spleen were similar (Figure 6E), histopathology revealed increased megakaryocytes and myeloid precursors in spleen, liver, and BM as well as increased BM fibrosis in p27CK− leukemic mice (Figure 6F). IF of MEFs and IHC of BM showed that in BCR-ABL1–positive p27CK− cells, the nuclear/cytoplasmic p27 ratio is not significantly different from EV-expressing p27CK− cells (supplemental Figure 12A-B). This might be because p27CK− mutant is unable to bind CDK2, which cannot be efficiently degraded, leading to a stabilization of nuclear p2722,38,39 that is not controlled by BCR-ABL1. Because nuclear p27CK− is nonfunctional, the leukemia-promoting effects of p27CK− can be ascribed to cytoplasmic p27, indicating that cytoplasmic p27 actively promotes leukemogenesis. To further understand how cytoplasmic p27 promotes oncogenesis, we performed RNAseq analysis of BCR-ABL1– and EV-transduced p27CK− and p27−/− BM cells (supplemental Figure 16, supplemental Table 5). We identified 54 genes with differential expression (DE) in p27CK− vs p27−/− cells transduced with BCR-ABL1 as compared with p27CK− vs p27−/− transduced with EV (supplemental Figure 16A). DE genes represent diverse Gene Ontology (GO) Biological Process categories (based on GO Slim summaries) such as genes associated with cell communication and localization (supplemental Figure 16B). Examination of the fine grain annotation for each biologic process allows identification of other related genes. For example, 7 genes in our DE list have GO biological process annotation for cell adhesion (Vcam1, Wisp2, Madcam1, Atp2c1, Mslnl, Frem2, S100a8). Kyoto Encyclopedia of Genes and Genomes enrichment analysis also showed significant enrichment of genes involved in cell adhesion (P < .01) and migration (P < .01), suggesting that perturbation of these cellular properties may confer a more aggressive phenotype. Examination of the gene candidates in the context of the functional annotations and known protein-protein interactions can guide future experiments to elucidate the underlying mechanisms.
Cytoplasmic p27 promotes BCR-ABL1–induced leukemia. (A) BM cells from p27−/− and p27CK− mice were transduced with BCR-ABL1 retrovirus, sorted by FACS for GFP+/Lin− cells and plated in methylcellulose in absence of cytokines. Myeloid colony formation of BCR-ABL1–transduced cells was assessed after 8 days. (B) Kaplan-Meier survival analysis of p27+/+ mice transplanted with BCR-ABL1–transduced BM cells from p27−/− and p27CK− mice. (C) WBC and PLT counts and (D) spleen and liver weights of leukemic mice were compared according to genotype. (E) Lineage distributions of GFP+ cells in the BM and spleen of leukemic mice: granulocytes (Gr1+CD11b+), B cells (CD19+), T cells (CD3+), and LKS cells (Lin−c-Kit+Sca1+). Error bars represent standard deviation. (F) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced BM cells from p27−/− and p27CK− mice. Scale bars represent 100 μm. In above experiments, **P < .010.
Cytoplasmic p27 promotes BCR-ABL1–induced leukemia. (A) BM cells from p27−/− and p27CK− mice were transduced with BCR-ABL1 retrovirus, sorted by FACS for GFP+/Lin− cells and plated in methylcellulose in absence of cytokines. Myeloid colony formation of BCR-ABL1–transduced cells was assessed after 8 days. (B) Kaplan-Meier survival analysis of p27+/+ mice transplanted with BCR-ABL1–transduced BM cells from p27−/− and p27CK− mice. (C) WBC and PLT counts and (D) spleen and liver weights of leukemic mice were compared according to genotype. (E) Lineage distributions of GFP+ cells in the BM and spleen of leukemic mice: granulocytes (Gr1+CD11b+), B cells (CD19+), T cells (CD3+), and LKS cells (Lin−c-Kit+Sca1+). Error bars represent standard deviation. (F) Representative histological sections of BM, liver, lung, and spleen from mice transplanted with BCR-ABL1–transduced BM cells from p27−/− and p27CK− mice. Scale bars represent 100 μm. In above experiments, **P < .010.
Discussion
Recent studies have implicated cytoplasmic p27 in oncogenesis, painting a complex picture of a protein originally thought to be a pure tumor suppressor.6,9,21 Here, we have dissected the 2 antagonistic activities of p27 in a well-defined cancer model. Because BCR-ABL1 is sufficient to induce CML, we reasoned that this disease is well suited to precisely characterize p27’s role in the process of leukemogenesis. We show that BCR-ABL1 promotes leukemia by coordinately disabling nuclear p27 tumor-suppressor functions in a kinase-dependent manner whereas simultaneously activating cytoplasmic oncogenic p27 functions in a kinase-independent fashion. These results are important in the context of restoring p27 function to treat CML and other malignancies.
Our experiments revealed that p27 localizes predominantly to the cytoplasm in CML cell lines and primary CD34+ cells (Figure 1A-B), expanding on previous observations.20,21,29 Although previous studies reported an increase in total and cytoplasmic p27 in CML vs normal CD34+ cells,20,21 and more cytoplasmic p27 in BP than CP,29 we found total p27 levels to be similar in both normal and CML cells and no differences between CP and BP (supplemental Figure 2B). The reason for these discrepancies is unclear, but it is possible that in vitro culture systems used previously influenced p27 expression. We performed all experiments on freshly isolated cells to minimize culture artifacts.
Imatinib inhibition of BCR-ABL1 induced an increase of nuclear p27 and decrease of SKP2 (supplemental Figure 2C-D). In contrast, cytoplasmic p27 levels remained unchanged upon BCR-ABL1 inhibition (Figure 1A-B), suggesting nuclear and cytoplasmic p27 abundance are regulated via distinct mechanisms, in a kinase-dependent and kinase-independent manner, respectively. Expression of kinase-inactive BCR-ABL1K271R in MEFs and 32Dcl3 cells demonstrated that BCR-ABL1 protein, but not its kinase activity, was required for localizing p27 to the cytoplasm (Figure 1C; supplemental Figures 3,4). In this context, Tomoda et al reported that BCR-ABL1 kinase upregulates the JAB1 complex to promote p27 degradation in the cytoplasm of some CML cell lines.28 Consistent with this, we observed an increase in cytoplasmic JAB1 in cells expressing cytoplasmic p27 (K562 and KYO-1) compared to Mo7ep210 cells, which express predominantly nuclear p27 (supplemental Figure 7). However, in our hands, imatinib had no impact upon JAB1 expression, which is at variance with Tomoda et al28 but consistent with the inability of imatinib to revert cytoplasmic p27 localization. The precise role of JAB1 in the regulation of p27 localization and stabilization remains to be determined.
We also investigated multiple BCR-ABL1 mutants and various inhibitors of key signal transduction pathways, including PI3K, MEK, and SRC, for their ability to influence cytoplasmic p27 (supplemental Tables 1,2; supplemental Figures 5,6). However, none of these interventions had a significant influence on cytoplasmic p27 expression. These data are at odds with a recent study reporting that cytoplasmic localization of p27 requires phosphorylation of BCR-ABL1 on Y177, activation of PI3K/AKT, and phosphorylation of p27 on T157.21 The discrepancy compared with our data may reflect the lack of specificity of the phospho-p27T157 antibody (R&D Systems) used in this and other studies (supplemental Figure 17). Moreover, we demonstrate that BCR-ABL1 induces cytoplasmic p27 mislocalization in murine cells (Figure 1C, supplemental Figures 3,4). As T157 of p27 is not conserved between humans and mice,40 this unequivocally shows that T157 is dispensable for cytoplasmic p27 localization. Given BCR-ABL1’s ability to form multiprotein complexes, we also tested whether p27 and BCR-ABL1 associate, but failed to detect p27 in cytoplasmic BCR-ABL1 immunoprecipitates from K562 cells and vice versa (data not shown). Moreover, p27 was not identified in a comprehensive proteomic analysis of BCR-ABL1–interacting proteins.41 Altogether, our data show that a kinase-independent activity of BCR-ABL1 drives cytoplasmic mislocalization of p27, but the precise pathways mediating the shift remain elusive.
Previous studies postulated that the key effect of cytoplasmic p27 mislocalization is sequestration away from nuclear Cdk2 complexes, preventing p27 from inhibiting cell-cycle progression, but these reports did not clarify whether cytoplasmic p27 has an oncogenic function in CML.20,21 In contrast, we demonstrate that downregulation of p27 has increased apoptosis in CML cells with predominantly cytoplasmic p27 (Figure 2C), consistent with a prosurvival activity that is independent of the nuclear p27 pool. Conversely, overexpression of cytoplasmic-only p27 in CML cells promoted cell growth (Figure 2D). These data and similar observations in some solid tumors identify cytoplasmic p27 as a legitimate therapeutic target.8,9
To dissect the various p27 activities in vivo, we took advantage of mouse strains with specific p27 mutations. Given the contrasting effects of cytoplasmic p27 (tumor promotion by inhibition of apoptosis) and nuclear p27 (tumor suppression by inhibition of cell-cycle progression), we first established the net function of p27 in leukemogenesis. Absence of p27 from BCR-ABL1–expressing leukemia cells promoted leukemogenesis as evidenced by reduced survival (Figure 3E-F). Deletion of 1 p27 allele produced an intermediate phenotype (Figure 3G-H), indicating p27 functions as a haploinsufficient tumor suppressor in CML, similar to its role in solid cancer.5,42,43
Having established that the net in vivo function of p27 in CML is tumor-suppressive, we separated the effects of the nuclear and cytoplasmic pools. Disease was attenuated in recipients of BCR-ABL1–infected p27T187A marrow (Figure 4). Analysis of BCR-ABL1–transduced MEFs and p27T187A leukemic mice marrow showed a significantly increased nuclear/cytoplasmic p27 ratio in BCR-ABL1–expressing p27T187A cells compared with p27+/+ controls (supplemental Figure 12). Compared with expression of EV, BCR-ABL1 expression reduced the nuclear/cytoplasmic ratio of p27T187A in MEFs, but not murine BM. No change in the nuclear/cytoplasmic ratio of p27 was observed in either tissue in case of p27CK− or p27S10A. The discrepancy between MEFs and BM may reflect the fact that, unlike MEFs, BM sections are not exclusively BCR-ABL1, which may reduce observable differences. In the case of p27CK−, the lack of a BCR-ABL1–induced change in the ratio may reflect its inability to bind CDK2, whereas S10A may be critical to the ability of BCR-ABL1 to promote cytoplasmic localization. For example, BCR-ABL1 may activate human kinase interacting stathmin to phosphorylate p27 at S10.37 This activation would have to occur in a BCR-ABL1 kinase-independent manner. Additional experimentation will be required to clarify this question. Overall, our results suggest that SKP2-mediated nuclear p27 degradation is relevant to CML leukemogenesis and is consistent with our previous report of prolonged survival when SKP2 is absent from the leukemia cells.18 The fact that p27T187A attenuates CML to a lesser degree than SKP2 deletion argues that SCFSKP2 has additional targets with tumor-suppressor function. Another possibility to explain the relatively subtle effects of inactivating p27T187-dependent nuclear degradation on leukemogenesis is that preserved oncogenic activity of cytoplasmic p27 counteracts effects of stabilizing nuclear p27. This would also explain why survival differences between p27T187A and p27+/+ leukemia are less pronounced (Figure 4) compared with p27−/− vs p27+/+ leukemia (Figure 3E-F). Additional evidence to support this comes from the p27S10A mutant, in which p27 is retained in the nucleus as evidenced by analysis of BCR-ABL1–transduced p27S10A MEFs and marrow (supplemental Figure 12A-B), presumably by reducing shuttling of p27 to the cytoplasm.22,35 Mice transplanted with BCR-ABL1–infected p27S10A marrow survived significantly longer than recipients of p27+/+ marrow (Figure 5B). These results are in agreement with the observation that p27S10A mice are more resistant to urethane-induced tumorigenesis than p27+/+ mice22 and suggest that increased nuclear and decreased cytoplasmic p27 attenuate BCR-ABL1–induced leukemia. To distinguish between these 2 possibilities and to assay cytoplasmic p27 function in isolation, we used p27CK− mice in which the nuclear cyclin/Cdk inhibitory function of p27 is abolished, whereas cytoplasmic functions are maintained.22 Compared with recipients of BCR-ABL1–infected p27−/− cells, mice receiving BCR-ABL1–infected p27CK− cells exhibited shortened survival and more extensive organ infiltration by leukemia cells (Figure 6). Because p27CK− lacks nuclear Cdk-inhibitory function, it follows that the greater aggressiveness of p27CK− leukemia emanates from an active contribution of cytoplasmic p27 to leukemogenesis. Furthermore, RNA-Seq analysis shows DE of several genes in p27CK− vs p27−/− cells transduced with BCR-ABL1 as compared with p27CK− vs p27−/− transduced with EV, including genes associated with migration and adhesion, which is consistent with the more extensive organ infiltration observed in p27CK− CML. Given the diversity of DE genes, other mechanisms likely are involved (supplemental Figure 16). One such possibility is that Cdk activity may be increased in p27CK− cells expressing BCR-ABL1. However, as it was previously shown that Cdk2 activity is similarly enhanced in p27CK− and p27−/− cells, we consider this unlikely.6
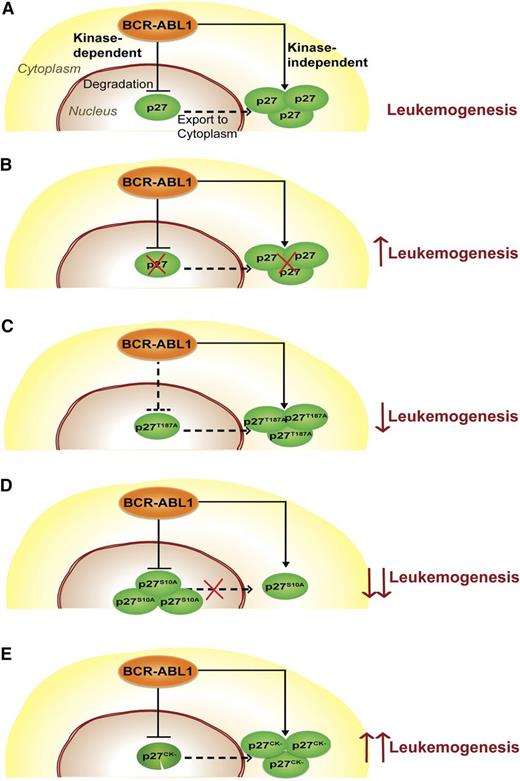
Our results fit a model in which BCR-ABL1 subverts p27 function by 2 independent mechanisms (Figure 7). A kinase-dependent pathway activates SCFSKP2, enhancing degradation of nuclear p27, thereby undermining its Cdk inhibitory/tumor suppressor activity. A kinase-independent pathway increases cytoplasmic p27 abundance, promoting survival.
Schematic describing the function of p27 in CML. (A) p27 distribution in CML cells. BCR-ABL1 degrades nuclear p27 in a kinase-dependent manner and promotes its cytoplasmic localization in a kinase-independent manner. (B) Complete loss of p27 (p27-null mutant). The absence of p27 promotes leukemogenesis, consistent with a net tumor-suppressive function of p27. (C) Nuclear stabilization of p27 (p27T187A mutant). Stabilization of nuclear p27 as a result of the p27T187A mutation only moderately attenuates leukemia because the oncogenic effect of cytoplasmic p27 persists. (D) Decreased p27 export to the cytoplasm (p27S10A mutant). Reduced cytoplasmic p27 and increased nuclear p27 retention due to the p27S10A mutation significantly reduces leukemogenesis of p27S10A mice. (E) Functional cytoplasmic and nonfunctional nuclear p27 (p27CK− mutant). The presence of functional cytoplasmic p27 and the complete absence of functional nuclear p27 in leukemic cells as a result of the p27CK− mutation promote leukemogenesis. As we do not provide direct evidence for trafficking of nuclear p27 to the cytoplasm, a dashed line was used to suggest nuclear-cytoplasmic shuttling of p27.
Schematic describing the function of p27 in CML. (A) p27 distribution in CML cells. BCR-ABL1 degrades nuclear p27 in a kinase-dependent manner and promotes its cytoplasmic localization in a kinase-independent manner. (B) Complete loss of p27 (p27-null mutant). The absence of p27 promotes leukemogenesis, consistent with a net tumor-suppressive function of p27. (C) Nuclear stabilization of p27 (p27T187A mutant). Stabilization of nuclear p27 as a result of the p27T187A mutation only moderately attenuates leukemia because the oncogenic effect of cytoplasmic p27 persists. (D) Decreased p27 export to the cytoplasm (p27S10A mutant). Reduced cytoplasmic p27 and increased nuclear p27 retention due to the p27S10A mutation significantly reduces leukemogenesis of p27S10A mice. (E) Functional cytoplasmic and nonfunctional nuclear p27 (p27CK− mutant). The presence of functional cytoplasmic p27 and the complete absence of functional nuclear p27 in leukemic cells as a result of the p27CK− mutation promote leukemogenesis. As we do not provide direct evidence for trafficking of nuclear p27 to the cytoplasm, a dashed line was used to suggest nuclear-cytoplasmic shuttling of p27.
Results from our study may have implications for CML therapy. Most CML patients treated with tyrosine kinase inhibitors (TKIs) harbor residual leukemia and experience a recurrence of disease upon TKI discontinuation, a situation referred to as disease persistence.44,45 In vitro studies have shown that primitive CML cells survive despite BCR-ABL1 kinase inhibition, suggesting persistence is BCR-ABL1 kinase-independent.44,46 Thus, it is conceivable that continued inhibition of apoptosis by cytoplasmic p27 despite TKI inhibition of BCR-ABL1 contributes to disease persistence in patients.
The fact that p27 expression is decreased in many types of cancer has sparked interest in developing agents to stabilize p27.4,47-49 Most of these strategies are focused on reducing p27 degradation.50-54 For example, bortezomib, a proteasome inhibitor effective in multiple myeloma, stabilizes total p27.55-57 Recently, highly selective inhibitors that occupy the p27 binding pocket at the SKP2-CKS1 interface of the SCFSKP2 complex were developed. These inhibitors prevent ubiquitylation and degradation of p27, reducing growth of cells with high SKP2 and low p27 expression.58 Thus, it may be possible to use small-molecule inhibitors to restore p27 expression in a specific fashion. In support of this approach, our results suggest that global stabilization of p27 should have a net tumor-suppressive effect. However, collateral promotion of cytosolic p27 accumulation could have undesired oncogenic effects that offset the benefits of re-establishing nuclear p27, suggesting that simultaneous inhibition of cytoplasmic transport will be required to fully exploit the potential of normalizing p27 function to treat CML and other cancers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sarah Bowden for administrative support. They also thank Dr Robert P. Searles (Director, Massively Parallel Sequencing Shared Resource) and Dr Christina Harrington (Director, OHSU Gene Profiling Shared Resources) for helping them with gene expression analysis.
This work was supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) 5K99 CA151670 02 (A.A.), 4R00CA151670-03 (A.A.), Lady Tata Memorial Trust (A.A.), Knight Career Development Award (A.A.), HL082978-01 (M.W.D.), CA04963920 (M.W.D.), NIH/NCI 1R01CA178397-01 (M.W.D., T.O.), and P01CA049639 (J.E.C., M.W.D.), Leukemia & Lymphoma Society 7036-01 (M.W.D.), and P30CA042014 (Huntsman Cancer Institute). B.J.D. is a Howard Hughes Medical Institute Investigator and is also supported by NIH/NCI MERIT award R37CA065823. A.A. is also supported by Collins Foundation, Knight Pilot Project, and Friends of Doernbecher grants. A.B. is supported by grants from the Fondation ARC pour la Recherches ur le Cancer, Ligue Nationale Contre le Cancer, and Institut National du Cancer.
Authorship
Contribution: A.A., R.J.M., A.B., D.H.L., A.G.F., N.D., M.M.L., S.M., S.J., A.C., and K.B.V. performed experiments; A.A. and M.W.D. designed the research, analyzed results, and wrote the paper; T.O., C.A.E., J.M.R., and B.J.D. provided critical feedback; and J.E.C., E.F., and J.M.R. provided vital reagents.
Conflict-of-interest disclosure: M.W.D. is on the paid advisory board and is a paid consultant for Bristol-Myers Squibb (BMS), ARIAD Pharmaceuticals, Novartis, Incyte, and Pfizer. M.W.D. also receives research funding from BMS, Novartis, Celgene, Genzyme, and Gilead. Oregon Health & Science University (OHSU) has clinical trial contracts with Novartis and BMS to pay for patient costs, nurse and data manager salaries, and institutional overhead. B.J.D. does not derive salary, nor does his laboratory receive funds, from these contracts. OHSU and B.J.D. have a financial interest in MolecularMD. Technology used in some of these studies has been licensed to MolecularMD by OHSU. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. The remaining authors declare no competing financial interests.
Correspondence: Michael W. Deininger, Huntsman Cancer Institute, 2000 Circle of Hope, Room 4261, Salt Lake City, UT 84112-5550; e-mail: michael.deininger@hci.utah.edu; and Anupriya Agarwal, Knight Cancer Institute, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Mail Code L592/BRB553, Portland, OR 97239; e-mail: agarwala@ohsu.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal