Abstract
Regulatory T-cells (Tregs) play a critical role in preventing autoimmune and alloimmune reactions, including graft-versus-host disease (GVHD). Two recent clinical trials demonstrated that in patients undergoing hematopoietic stem cell transplantation, adoptive transfer of Tregs significantly reduced the incidence of grades II-IV GVHD. While Tregs significantly reduced GVHD severity, they did not eliminate GVHD. One potential way to augment Treg-mediated inhibition of GVHD is to increase Treg suppressive potency. We showed previously that Treg-specific inhibition of protein kinase C-theta (PKC-θ) enhances Treg function (Science 328:372, 2010). However, it is unclear whether PKC-θ inhibition can boost Treg function in a systemic inflammatory condition like GVHD. Furthermore, the mechanism by which PKC-θ inhibition augments Treg function is unknown. In this study, we address these unanswered questions.
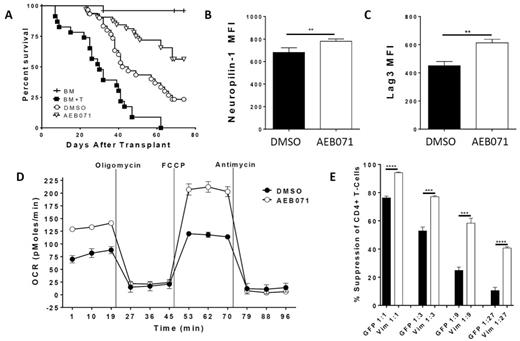
Using a mouse MHC class I/II disparate acute GVHD model, we found that freshly isolated Tregs treated for 30 minutes with 10uM of the clinically available PKC-θ inhibitor AEB071 suppressed GVHD mortality (Fig 1A) and severity significantly better than DMSO treated Tregs. As Tregs exert much of their protective effect against GVHD early in the course of the disease, we analyzed proliferation of GVHD-causing conventional T-cells (Tcon) on D4 after transplant. We observed a significant reduction in Tcon proliferation in mice given AEB071 treated Tregs compared to DMSO treated Tregs. We then performed multi-photon microscopy on D4 after transplant using TEα-GFP Tcon, CD11c-eYFP antigen presenting cells (APCs) and wild-type Tregs. Compared to DMSO, AEB071 treated Tregs significantly increased Tcon velocity and displacement from APCs. Increased velocity and displacement are indicative of decreased Tcon-APC interactions, suggesting reduced priming when AEB071 Tregs are present.
Mechanistically, AEB071 vs DMSO treatment of Tregs resulted in augmented expression of the suppressive molecules Neuropilin-1 (Nrp1) and Lymphocyte activation gene 3 (Lag3) after in vitro activation (Fig 1B, C) and in Tregs isolated from acute GVHD mice. Antibody blockade of Nrp1 and Lag3 in in vitro transwell suppression assays reduced the effect of AEB071 treatment, suggesting that these molecules may play a role in enhancing Treg function after PKC-θ inhibition. Flow cytometry analysis of phosphorylated proteins in activated Tregs revealed that PKC-θ inhibition resulted in reduced phosphorylation of the mTORC2 target FoxO3a, but not mTORC1 targets S6 and 4E-BP1. In addition, the mTORC2-specific phosphorylation site on Akt, serine 473, was reduced, whereas the mTORC1-specific site, threonine 308, was unaltered. Together, these data suggest reduced mTORC2 activity. Reduced phosphorylation increases Foxo3a nuclear translocation, which may result in increased Nrp1 and Lag3 expression, since Foxo3a has binding sites in both gene promoters. As both mTORC1 and 2 are involved in T-cell metabolism, we investigated the effect of AEB071 treatment on Treg oxygen consumption rate (OCR). Compared to DMSO, AEB071 treatment significantly increased Treg baseline and maximal OCRs after activation (Fig 1D). Increased OCR has been associated with increased Treg function.
To identify additional alterations in phosphorylated proteins after PKC-θ inhibition, we performed a phosphoproteomic screen using in vitro expanded human Tregs treated with AEB701 or DMSO. We identified significant alterations in phosphorylation sites on 72 proteins, including reduced phosphorylation of an adaptor molecule that links PKC-θ to the intermediate filament vimentin. We found that vimentin is highly upregulated in Tregs compared to Tcon and that in Tregs, vimentin interacts with PKC-θ after activation. AEB071 treatment reduced the interaction between vimentin and PKC-θ. As with AEB071 treatment, Vimentin siRNA significantly increased Treg suppression in vitro compared to control transfected Tregs (Fig 1E), and augmented expression of Nrp1 and Lag3. AEB071 treatment of vimentin siRNA transfected Tregs did not further augment Treg function, suggesting an overlapping mechanism.
In summary, our data demonstrate that PKC-θ interacts with mTORC2 and vimentin to modulate multiple aspects of Treg function, and that a brief incubation of Tregs with a PKC-θ inhibitor may be a viable method to enhance the efficacy of Treg therapeutics.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal