Abstract
Background: Gilteritinib improves response rates and overall survival (OS) compared with chemotherapy in patients (pts) with relapsed/refractory (R/R) FLT3-mutated acute myeloid leukemia (AML). For older pts with newly diagnosed (ND) FLT3-mutated AML who are unfit for intensive chemotherapy, azacitidine plus venetoclax is the current standard of care, although remission durations are relatively short, largely due to FLT3-driven relapses. We therefore sought to evaluate the triplet regimen of azacitidine, venetoclax and gilteritinib for older pts with ND FLT3-mutated AML, as well as for those of all ages with R/R FLT3-mutated AML.
Methods: In this phase I/II study, pts with either R/R FLT3-mutated AML or high-risk MDS/CMML or pts with ND FLT3-mutated AML who were unsuitable for intensive chemotherapy were eligible. FLT3-ITD and/or TKD mutations were allowed. Pts were required to have a performance status ≤3, total bilirubin ≤2.5 x ULN, ALT/AST ≤3 x ULN, and creatinine clearance ≥30 mL/min. In cycle 1, pts received azacitidine 75 mg/m 2 SC/IV on days 1-7, venetoclax on days 1-28, and gilteritinib on days 1-28. Gilteritinib dose ranged from 80mg to 120mg daily during the phase I dose escalation (3+3 design). Bone marrow was performed on day 14 and if blasts <5% or aplastic marrow, then both venetoclax and gilteritinib were held (ND cohort) or only venetoclax was held (R/R cohort). For cycles 2 and beyond, azacitidine 75 mg/m 2 SC/IV was given for 5-7 days, venetoclax was given for 7-14 days and gilteritinib was given continuously.
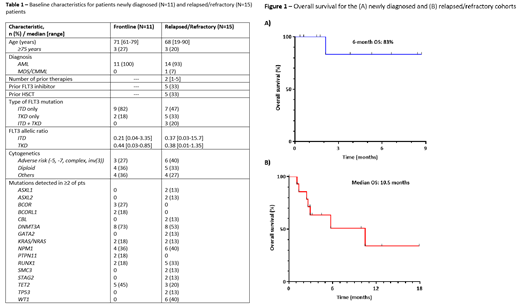
Results: Between 12/2019 and 7/2021, 26 pts were treated (11 ND pts and 15 R/R pts). Baseline characteristics are shown in Table 1. The median age for the ND and R/R cohorts were 71 years (range, 61-79) and 68 years (range, 19-90), respectively. In the ND cohort, 9 (82%) had a FLT3-ITD and 2 (18%) had a FLT3 TKD mutation; in the R/R cohort, 7 (47%) had a FLT3-ITD, 5 (33%) had a FLT3-TKD, and 3 (20%) had both mutations. Among pts in the R/R cohort, the median number of prior therapies was 2 (range, 1-5), 6 (40%) had poor risk cytogenetics, 5 (33%) had undergone prior hematopoietic stem cell transplant (HSCT), and 5 (33%) had received prior FLT3 inhibitor (including 1 pt who had received prior gilteritinib).
Ten R/R pts were treated in the phase I cohort (6 at 80mg of gilteritinib and 4 at 120mg). No DLTs were observed at the 80mg daily dosing. Among 3 pts in the 120mg cohort who were evaluable for DLTs, 1 achieved MLFS but had prolonged grade 4 myelosuppression that met DLT criteria. Due to good clinical activity and superior count recovery observed with the 80mg dosing (with 3 of 6 pts [50%] in the phase I cohort achieving CR/CRp), the 80mg daily dose was chosen as the phase II dose for further study.
In the entire R/R cohort, the median number of cycles received was 2 (range, 1-4). The overall response rate (CR+CRi+MLFS) was 67%. One pt (7%) achieved CR as best response, 3 (20%) achieved CRi, and 6 (40%) achieved MLFS. An additional pt with extramedullary-only disease achieved PR. Four pts (27% of the entire R/R cohort, 44% of responding pts) proceeded to HSCT. Among the 10 responding pts, 4 have relapsed (1 of whom was FLT3 negative), 2 died in morphologic remission, and 4 are still alive without relapse. The 30-day and 60-day mortality rates were 0% and 13%, respectively. With a median follow-up of 9.9 months in the R/R cohort, the median duration of response was 9.0 months and the median OS was 10.5 months (Figure 1A).
In the ND cohort, the median number of cycles received was 2 (range, 1-6). All pts responded and all achieved marrow remission by day 14. Eight pts (73%) achieved CR as best response, 1 (9%) achieved CRi, and 2 (18%) achieved MLFS; the 3 pts with CRi/MLFS are still recovering from cycle 1 at the time of this report. Two pts (18%) proceeded to HSCT. With a median follow-up of 3.8 months (range, 0.5-8.7 months), no pts have relapsed and 1 pt died (Figure 1B). This pt achieved MRD-negative CR and died 9 weeks into therapy due to sepsis.
Conclusions: The combination of azacitidine, venetoclax and gilteritinib was effective in pts with FLT3-mutated AML. Gilteritinib dosing at 80mg daily was associated with a better safety/efficacy profile and was selected for future study; however, even with this lower dose myelosuppression was common and required attenuation of azacitidine and venetoclax. This combination appears particularly encouraging in the frontline setting where the response rate was 100% with no relapses observed to date.
Short: Astellas: Research Funding; AstraZeneca: Consultancy; Novartis: Honoraria; Jazz Pharmaceuticals: Consultancy; NGMBio: Consultancy; Takeda Oncology: Consultancy, Research Funding; Amgen: Consultancy, Honoraria. DiNardo: Celgene, a Bristol Myers Squibb company: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Forma: Honoraria, Research Funding; AbbVie: Consultancy, Research Funding; GlaxoSmithKline: Membership on an entity's Board of Directors or advisory committees; Foghorn: Honoraria, Research Funding; Notable Labs: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; ImmuneOnc: Honoraria, Research Funding; Agios/Servier: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Novartis: Honoraria. Daver: Amgen: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; FATE Therapeutics: Research Funding; Hanmi: Research Funding; Genentech: Consultancy, Research Funding; Trovagene: Consultancy, Research Funding; Glycomimetics: Research Funding; Sevier: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Novimmune: Research Funding; Abbvie: Consultancy, Research Funding; Astellas: Consultancy, Research Funding; Gilead Sciences, Inc.: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; ImmunoGen: Consultancy, Research Funding; Novartis: Consultancy; Jazz Pharmaceuticals: Consultancy, Other: Data Monitoring Committee member; Dava Oncology (Arog): Consultancy; Celgene: Consultancy; Syndax: Consultancy; Shattuck Labs: Consultancy; Agios: Consultancy; Kite Pharmaceuticals: Consultancy; SOBI: Consultancy; STAR Therapeutics: Consultancy; Karyopharm: Research Funding; Newave: Research Funding. Yilmaz: Daiichi-Sankyo: Research Funding; Pfizer: Research Funding. Kadia: Genfleet: Other; Astellas: Other; AstraZeneca: Other; Cellonkos: Other; Sanofi-Aventis: Consultancy; Pulmotech: Other; Pfizer: Consultancy, Other; Liberum: Consultancy; BMS: Other: Grant/research support; Jazz: Consultancy; Genentech: Consultancy, Other: Grant/research support; Cure: Speakers Bureau; Ascentage: Other; Novartis: Consultancy; AbbVie: Consultancy, Other: Grant/research support; Aglos: Consultancy; Dalichi Sankyo: Consultancy; Amgen: Other: Grant/research support. Issa: Novartis: Consultancy, Research Funding; Syndax Pharmaceuticals: Research Funding; Kura Oncology: Consultancy, Research Funding. Sasaki: Novartis: Consultancy, Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Membership on an entity's Board of Directors or advisory committees. Borthakur: Takeda: Membership on an entity's Board of Directors or advisory committees; ArgenX: Membership on an entity's Board of Directors or advisory committees; Ryvu: Research Funding; Astex: Research Funding; University of Texas MD Anderson Cancer Center: Current Employment; Protagonist: Consultancy; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy. Jabbour: Amgen, AbbVie, Spectrum, BMS, Takeda, Pfizer, Adaptive, Genentech: Research Funding. Konopleva: F. Hoffmann-La Roche: Consultancy, Honoraria, Other: grant support; Forty Seven: Other: grant support, Research Funding; Calithera: Other: grant support, Research Funding; Genentech: Consultancy, Honoraria, Other: grant support, Research Funding; Reata Pharmaceuticals: Current holder of stock options in a privately-held company, Patents & Royalties: intellectual property rights; Novartis: Other: research funding pending, Patents & Royalties: intellectual property rights; Rafael Pharmaceuticals: Other: grant support, Research Funding; AstraZeneca: Other: grant support, Research Funding; Stemline Therapeutics: Research Funding; Eli Lilly: Patents & Royalties: intellectual property rights, Research Funding; KisoJi: Research Funding; Sanofi: Other: grant support, Research Funding; AbbVie: Consultancy, Honoraria, Other: Grant Support, Research Funding; Agios: Other: grant support, Research Funding; Ascentage: Other: grant support, Research Funding; Ablynx: Other: grant support, Research Funding; Cellectis: Other: grant support. Kantarjian: Ipsen Pharmaceuticals: Honoraria; NOVA Research: Honoraria; Amgen: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Ascentage: Research Funding; BMS: Research Funding; Daiichi-Sankyo: Research Funding; Immunogen: Research Funding; Jazz: Research Funding; Pfizer: Honoraria, Research Funding; KAHR Medical Ltd: Honoraria; Astra Zeneca: Honoraria; Astellas Health: Honoraria; Novartis: Honoraria, Research Funding; Aptitude Health: Honoraria; Precision Biosciences: Honoraria; Taiho Pharmaceutical Canada: Honoraria. Ravandi: Agios: Honoraria, Research Funding; Astex: Honoraria, Research Funding; Jazz: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Prelude: Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AstraZeneca: Honoraria; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Xencor: Honoraria, Research Funding; Taiho: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Novartis: Honoraria; Syros Pharmaceuticals: Consultancy, Honoraria, Research Funding.
Gilteritinib for frontline treatment of FLT3-mutated AML
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal