Key Points
The Bor-HDM (200 mg/m2) conditioning regimen is not superior to HDM alone in de novo multiple myeloma.
HDM (200 mg/m2) remains the standard-of-care conditioning for transplantation in multiple myeloma.
Abstract
High-dose melphalan (HDM) and transplantation are recommended for eligible patients with multiple myeloma. No other conditioning regimen has proven to be more effective and/or safer. We previously reported in a phase 2 study that bortezomib can safely and effectively be combined with HDM (Bor-HDM), with a 32% complete response (CR) rate after transplantation. These data supported a randomized phase 3 trial. Randomization was stratified according to risk and response to induction: 300 patients were enrolled, and 154 were allocated to the experimental arm (ie, arm A) with bortezomib (1 mg/m2 intravenously [IV]) on days −6, –3, +1, and +4 and melphalan (200 mg/m2 IV) on day –2. The control arm (ie, arm B) consisted of HDM alone (200 mg/m2 IV). There were no differences in stringent CR + CR rates at day 60 posttransplant (primary end point): 22.1% in arm A vs 20.5% in arm B (P = .844). There were also no differences in undetectable minimum residual disease rates: 41.3% vs 39.4% (P = .864). Median progression-free survival was 34.0 months for arm A vs 29.6 months for arm B (adjusted HR, 0.82; 95% CI, 0.61-1.13; P = .244). The estimated 3-year overall survival was 89.5% in both arms (hazard ratio, 1.28; 95% CI, 0.62-2.64; P = .374). Sixty-nine serious adverse events occurred in 18.7% of Bor-HDM–treated patients (vs 13.1% in HDM-treated patients). The proportion of grade 3/4 AEs was similar within the 2 groups (72.0% vs 73.1%), mainly (as expected) blood and gastrointestinal disorders; 4% of patients reported grade 3/4 or painful peripheral neuropathy in arm A (vs 1.5% in arm B). In this randomized phase 3 study, a conditioning regimen with Bor-HDM did not improve efficacy end points or outcomes compared with HDM alone. The original trial was registered at www.clinicaltrials.gov as #NCT02197221.
Introduction
High-dose therapy (HDT) followed by autologous stem cell transplantation (ASCT) remains a gold standard for patients newly diagnosed with multiple myeloma (MM) eligible to undergo the procedure. For >25 years, clinical trials have reported the superiority of HDT over conventional chemotherapy in terms of response rate, progression-free survival (PFS),1-4 and/or overall survival (OS).5-7 The outcome after transplantation is related to the quality of response, and achieving at least a complete response (CR) is a major prognostic factor for long-term survival.8-12 Proteasome inhibitors and/or IMiDs (Bristol Myers Squibb) have considerably improved response rates, and 20% to 40% of patients can achieve CR with triplet induction regimen (excluding monoclonal antibodies) followed by transplantation.13-20 HDM (200 mg/m2) is the most commonly used conditioning regimen before transplantation. Alternatives to HDM have been explored, but although more intense conditioning regimens could result in higher CR rates, combinations with HDM frequently induced additional hematologic and non-hematologic toxicities and generally failed to increase survival.21-25
Bortezomib is a potent, selective, and reversible proteasome inhibitor.26 Synergistic apoptotic effects have been reported both in vitro27 and in vivo28,29 with melphalan. The combination of bortezomib and HDM (Bor-HDM) was an attractive approach to improve the efficacy of the conditioning regimen. This association was expected to be safe, with no overlapping toxicities. The Intergroupe Francophone du Myélome (IFM) previously reported a phase 2 trial of this Bor-HDM conditioning regimen.30 This preparative regimen was well tolerated, with no treatment-related mortality or increased toxicity. Engraftment was not affected by the addition of bortezomib. Overall, 70% of patients achieved very good partial response (VGPR) or better, with at least 32% of patients in CR after a single transplant, regardless of the type of induction therapy used at that time (vincristine-adriamycin-dexamethasone or bortezomib-dexamethasone). These data supported a phase 3 study, and the current prospective randomized trial was designed to compare the rate of CR or better at day 60 posttransplantation, before consolidation, and to assess whether the Bor-HDM conditioning regimen could effectively be superior to HDM alone in current transplant programs with triplet induction and consolidation therapies.
Methods
Eligibility criteria
Patients aged <66 years with symptomatic newly diagnosed MM could be included if they were eligible for transplantation and they had nonprogressive disease after induction therapy. Key non-inclusion criteria comprised: creatinine clearance ≤40 mL/min at time of transplant; serum total bilirubin level ≥1.5 × the upper limit of the normal range and serum aspartate/alanine aminotransferase levels ≥3.5 × the upper limit of the normal range; a left ventricular ejection fraction ≤40% and a pulmonary diffusing capacity ≤50% of predicted; a grade 3 or worse peripheral neuropathy (PNY) or grade 2 with pain; a significant comorbid disease (HIV infection, active hepatitis B virus or hepatitis C virus infection, clinically significant cardiac disease, uncontrolled hypertension, or diabetes); and a history of any other malignant disease. The institutional ethics committees approved the study, and all patients gave written informed consent before entering the study.
Study design
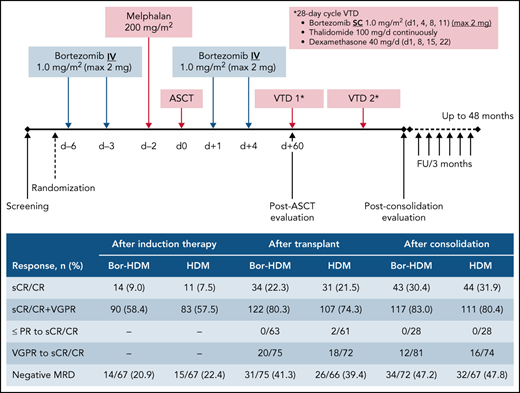
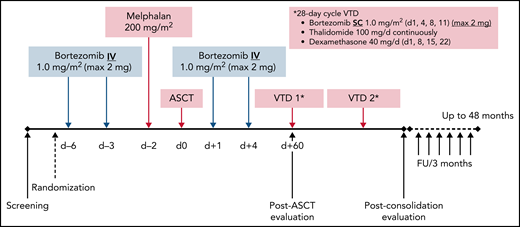
This multicenter, randomized, open-label, phase 3 study was designed in 2012 to compare the efficacy and safety of bortezomib in combination with HDM (Bor-HDM) vs HDM alone as a transplant conditioning regimen in patients newly diagnosed with MM. In experimental arm A, bortezomib at 1 mg/m2 was administered intravenously on days −6, –3, +1, and +4. HDM at 200 mg/m2 was administered intravenously over 30 minutes on day −2. Arm B consisted of HDM alone on day −2. Peripheral blood stem cells (minimum ≥2 × 106 CD34+ cells/kg) were infused on day 0. The Bor-HDM treatment schema is shown in Figure 1. Randomization was stratified according to International Staging System31 (I-II vs III), fluorescence in situ hybridization (FISH) analysis (standard vs high risk [del(17p) or t(4;14)] vs non-informative FISH) and response after induction (VGPR or better vs partial response/stable disease). All patients received standard supportive-care measures, including growth factor support, blood transfusions, and prophylactic or therapeutic antibiotics, according to local guidelines. Patients were discharged home after neutrophil (absolute neutrophil count ≥0.5 × 109/L for 3 consecutive days) and platelet (≥20 × 109/L without transfusion) recovery.
Bor-HDM conditioning regimen schema. d, day; FU, follow-up; max, maximum; IV, intravenously; SC, subcutaneously.
Bor-HDM conditioning regimen schema. d, day; FU, follow-up; max, maximum; IV, intravenously; SC, subcutaneously.
The choice of a bortezomib-based induction regimen was at the discretion of the investigator, with the following options: bortezomib, thalidomide, and dexamethasone (VTd); bortezomib, cyclophosphamide, and low-dose dexamethasone (CyBorD); or bortezomib, lenalidomide, and low-dose dexamethasone (RVd). Patients with non-progressive disease received two 28-day cycles of VTd as consolidation at day 60 posttransplant (subcutaneous bortezomib at 1 mg/m2 on days 1, 4, 8, and 11; oral thalidomide 100 mg/d continuously; and oral dexamethasone 40 mg weekly). No maintenance therapy was planned, as this was not authorized in France at that time. All patients were evaluated at day 60 post-ASCT, at the completion of 2 cycles of consolidation, and every 3 months until disease progression or end of trial.
Criteria for evaluation
The primary end point of the trial was achievement of stringent CR (sCR) or CR at day 60 post-ASCT based on investigator response assessment. Non-evaluable patients were considered as failure in achieving CR. The secondary end points were safety profile of the combination regimen, response at each time points, best response from randomization and overall response rates, PFS, and OS. Initial diagnostic and staging evaluations, initial therapies, and disease status at enrollment were documented for all patients. Myeloma testing included screening for chromosomal abnormalities by FISH analysis, serum β2-microglobulin and albumin levels, serum and urine electrophoresis plus immunofixation, and imaging studies. Toxicities were graded according to National Cancer Institute Common Toxicity Criteria of Adverse Events (version 4.03). Myeloma response and relapse definitions were based on the revised International Myeloma Working Group consensus criteria for response.32 Minimal residual disease (MRD) was assessed in bone marrow samples by multiparametric flow cytometry (MFC) at a sensitivity of at least 2 × 10−5 in patients in CR or VGPR; patients who failed to achieve at least VGPR were considered as MRD positive. All patients were followed up until disease progression, death, or the reference date (31 December 2018).
Statistical considerations
The sample size was determined assuming 40% CR or better in the HDM alone arm, and 60% in the Bor-HDM arm at day 60 posttransplant. Three hundred patients (150 per arm) were needed for reaching at least 90% statistical power, with a two-sided type I error level of 0.05, based on a normal-approximate z test, assuming that ∼7% of randomized patients may not be evaluable for CR at day 60. For efficacy end points, all analyses were performed on the intention-to-treat principle. A stratified Cochran-Mantel-Haenszel test was used to compare differences in response rates. Treatment effect size was provided by using adjusted hazard ratios (HRs) on stratification factors along with 95% confidence intervals (95% CIs). To explore homogeneity of treatment effects, subgroup analyses were conducted on prespecified baseline variables, and interactions between arm and covariate were tested. Fisher’s exact test or exact Pearson χ2 tests were used to compare response rates. PFS was defined as the time from randomization until either the first documentation of progressive disease or death from any cause. OS was defined as the time from randomization until death. Follow-up was estimated by using the reverse Kaplan-Meier method. Time-to-event end points were analyzed by using the Kaplan-Meier method, with a two-sided stratified log-rank test to compare the treatment arms. Toxicities and adverse events (AEs) were analyzed in the treated population (ie, patients who received at least 1 dose of the conditioning treatment). Comparisons were two-sided at a 5% statistical significance level. All statistical analyses were performed with SAS version 9.4 (SAS Institute, Inc.).
Results
Patients
Between 21 January 2015 and 21 September 2016, a total of 332 patients with de novo MM were screened and 300 were randomized to treatment in 47 IFM transplant centers. Patients were randomly assigned to receive Bor-HDM (arm A, n = 154) or HDM alone (arm B, n = 146) as a conditioning regimen before transplantation. Patients’ demographic and baseline characteristics are summarized in Table 1. FISH analysis was performed in 295 (98.3%) patients, including the presence of a 17p deletion in 17 patients (6.5%) and a t(4;14) translocation in 31 patients (11.7%). All patients received a bortezomib-based induction, and the vast majority (n = 262 [(87.3%]) received VTd. Other regimens included: CyBorD in 34 (11.3%) patients; RVd in 2 (0.7%) patients; and bortezomib-dexamethasone in 2 (0.7%) patients. Induction regimens and responses were well balanced between the 2 arms (see patient characteristics).
Baseline demographic and disease-related patient characteristics
| Characteristic . | Bor-HDM (n = 154) . | HDM (n = 146) . |
|---|---|---|
| Sex, Male/Female, n | 99/55 | 83/63 |
| Age, median (Q1-Q3), y | 58 (52-63) | 58 (52-62) |
| Isotype, n (%) | ||
| IgG | 93 (60.4) | 98 (67.1) |
| IgA | 38 (24.7) | 31 (21.2) |
| Light chain/others | 21/2 (13.6/1.3) | 16/1 (11.0/0.7) |
| ISS stage, n (%) | ||
| I, II | 89/43 (85.7) | 80/45 (85.6) |
| III | 22 (14.3) | 21 (14.4) |
| β2-Microglobulin, median (Q1-Q3), mg/L | 2.82 (2.15-4.30) | 3.00 (2.32-4.10) |
| Albumin, median (Q1-Q3), g/L | 40.9 (36.7-44.3) | 40.7 (36.8-44.1) |
| “CRAB” criterion, n (%) | ||
| Calcium elevation | 8 (5.2) | 8 (5.5) |
| Renal insufficiency | 2 (1.3) | 6 (4.1) |
| Anemia | 45 (29.2) | 44 (30.1) |
| Bone lesions | 129 (83.8) | 134 (91.8) |
| Cytogenetic risk, n/N (%) | ||
| Standard risk | 109/151 (72.2) | 106/144 (73.6) |
| High risk [del(17p) and/or t(4;14)] | 25/151 (16.6) | 22/144 (15.3) |
| Non-informative | 17/151 (11.3) | 16/144 (11.1) |
| del(17p) | 7/133 (5.3) | 10/128 (7.8) |
| t(4;14) | 18/136 (13.2) | 13/129 (10.1) |
| Frontline treatments, n (%) | ||
| VTd | 137 (89.0) | 125 (85.6) |
| VCD/CyBorD | 16 (10) | 18 (12.3) |
| Response after induction, n (%) | ||
| At least very good partial response | 90 (58.4) | 83 (56.8) |
| Partial response or stable disease | 64 (41.6) | 63 (43.2) |
| Characteristic . | Bor-HDM (n = 154) . | HDM (n = 146) . |
|---|---|---|
| Sex, Male/Female, n | 99/55 | 83/63 |
| Age, median (Q1-Q3), y | 58 (52-63) | 58 (52-62) |
| Isotype, n (%) | ||
| IgG | 93 (60.4) | 98 (67.1) |
| IgA | 38 (24.7) | 31 (21.2) |
| Light chain/others | 21/2 (13.6/1.3) | 16/1 (11.0/0.7) |
| ISS stage, n (%) | ||
| I, II | 89/43 (85.7) | 80/45 (85.6) |
| III | 22 (14.3) | 21 (14.4) |
| β2-Microglobulin, median (Q1-Q3), mg/L | 2.82 (2.15-4.30) | 3.00 (2.32-4.10) |
| Albumin, median (Q1-Q3), g/L | 40.9 (36.7-44.3) | 40.7 (36.8-44.1) |
| “CRAB” criterion, n (%) | ||
| Calcium elevation | 8 (5.2) | 8 (5.5) |
| Renal insufficiency | 2 (1.3) | 6 (4.1) |
| Anemia | 45 (29.2) | 44 (30.1) |
| Bone lesions | 129 (83.8) | 134 (91.8) |
| Cytogenetic risk, n/N (%) | ||
| Standard risk | 109/151 (72.2) | 106/144 (73.6) |
| High risk [del(17p) and/or t(4;14)] | 25/151 (16.6) | 22/144 (15.3) |
| Non-informative | 17/151 (11.3) | 16/144 (11.1) |
| del(17p) | 7/133 (5.3) | 10/128 (7.8) |
| t(4;14) | 18/136 (13.2) | 13/129 (10.1) |
| Frontline treatments, n (%) | ||
| VTd | 137 (89.0) | 125 (85.6) |
| VCD/CyBorD | 16 (10) | 18 (12.3) |
| Response after induction, n (%) | ||
| At least very good partial response | 90 (58.4) | 83 (56.8) |
| Partial response or stable disease | 64 (41.6) | 63 (43.2) |
ISS, International Staging System; Ig, immunoglobulin; VCD/CyBorD, bortezomib, cyclophosphamide and dexamethasone.
Study treatment exposure
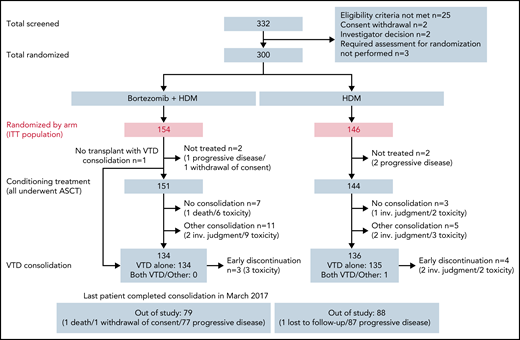
In the Bor-HDM arm, 151 (98%) of 154 patients received the planned conditioning regimen followed by transplantation (vs 98.5% in the HDM arm). Overall, 5 patients did not receive HDT because of progressive disease (n = 3), withdrawal of consent (n = 1), or stem cell growth defect (n = 1). One additional patient, allocated to the Bor-HDM arm, received only HDM because of grade 2 neuropathy at time of conditioning regimen. Figure 2 presents patient disposition. Regarding bortezomib administration, 147 (95.5%) patients received the 4 planned doses; 2 patients received only 3 doses and 1 patient only 2 doses. Dose reduction was due to AEs only in 1 patient. Ten patients did not receive further consolidation, including 7 patients (4.6%) in the Bor-HDM arm. Consolidation comprised VTd as planned for 2 cycles in 128 (83.1%) of 145 patients and 130 (89.0%) of 141 patients, respectively. Five patients received >2 cycles according to investigator judgment to improve responses: n = 3 (2.0%) patients after Bor-HDM and n = 2 (1.4%) patients after HDM alone, respectively. The remaining patients (n = 16) received a lenalidomide-based consolidation (median, 2 cycles; range, 2-24 cycles) essentially because of neuropathy.
Consolidated Standards of Reporting Trials diagram of patients’ disposition. inv., investigator; ITT, intention-to-treat.
Consolidated Standards of Reporting Trials diagram of patients’ disposition. inv., investigator; ITT, intention-to-treat.
Response evaluation
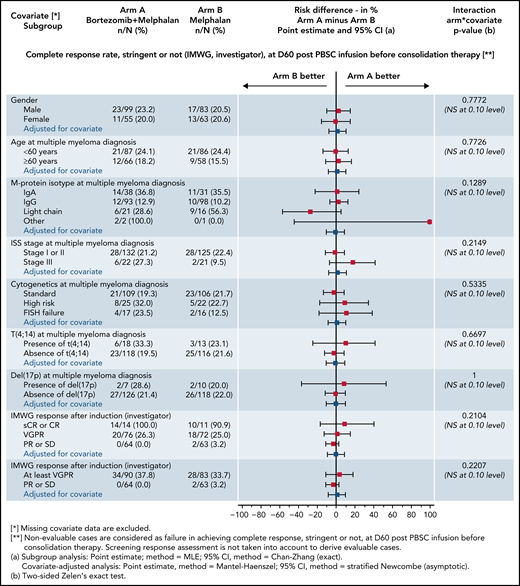
At day 60 posttransplant, there was no difference regarding sCR/CR rates (primary end point): 22.1% in the Bor-HDM arm vs 20.5% in the HDM arm (HR, 1.06; 95% CI, 0.56-2.03; P = .844). These results were homogeneous across strata for all the prespecified baseline covariates (Figure 3). There was no difference in undetectable MRD rates at day 60 posttransplant in patients with VGPR or better assessed by MFC (10−5): 41.3% vs 39.4%, respectively (P = .864). At the completion of consolidation, 30.4% of patients were in CR or better in the Bor-HDM arm vs 31.9% in the HDM arm (P = .626). Table 2 presents response rates at different time points and their improvement. There was no difference for best responses achieved: 84.4% VGPR or better and 44.8% CR or better in the Bor-HDM arm vs 82.9% VGPR or better and 42.5% CR or better in the HDM arm (P = .756 and P = .727, respectively). MRD at any time point during the program was undetectable in 39.5% of patients in arm A vs 38.4% (P = .896) in arm B.
Subgroup and covariate analyses with risk differences of sCR/CR rates at day 60 posttransplant (intention-to-treat population). IgA, immunoglobulin A; IMWG, International Myeloma Working Group; ISS, International Staging System; NS, not significant; PBSC, peripheral blood stem cells; PD, progressive disease; PR, partial response; SD, stable disease.
Subgroup and covariate analyses with risk differences of sCR/CR rates at day 60 posttransplant (intention-to-treat population). IgA, immunoglobulin A; IMWG, International Myeloma Working Group; ISS, International Staging System; NS, not significant; PBSC, peripheral blood stem cells; PD, progressive disease; PR, partial response; SD, stable disease.
Summary of responses and improvements (per protocol)
| Response . | After induction therapy . | After ASCT . | After consolidation . | Best response . | ||||
|---|---|---|---|---|---|---|---|---|
| Bor-HDM (n = 154) . | HDM (n = 146) . | Bor-HDM (n = 152*) . | HDM (n = 144) . | Bor-HDM (n = 141) . | HDM (n = 138) . | Bor-HDM (n = 152*) . | HDM (n = 146) . | |
| sCR | 9 (5.8) | 4 (2.7) | 16 (10.5) | 19 (13.2) | 27 (19.1) | 31 (22.5) | 45 (29.6) | 45 (30.8) |
| CR | 5 (3.2) | 7 (4.8) | 18 (11.8) | 12 (8.3) | 16 (11.3) | 13 (9.4) | 24 (15.8) | 17 (11.6) |
| VGPR | 76 (49.4) | 72 (49.3) | 88 (57.9) | 76 (52.8) | 74 (52.5) | 67 (48.6) | 61 (40.1) | 59 (40.4) |
| Partial response | 55 (35.7) | 54 (37.0) | 29 (19.1) | 31 (21.5) | 22 (15.6) | 22 (15.9) | 21 (13.8) | 20 (13.7) |
| Stable disease | 9 (5.8) | 9 (6.2) | 0 | 4 (2.8) | 0 | 3 (2.2) | 0 | 3 (2.1) |
| PD | 0 | 0 | 1 (0.7) | 2 (1.4) | 2 (1.4) | 2 (1.4) | 1 (0.7)† | 2 (1.4)† |
| Negative MRD‡ | 14/67 (20.9) | 15/67 (22.4) | 31/75 (41.3) | 26/66 (39.4) | 34/72 (47.2) | 32/67 (47.8) | 47/90 (52.2) | 47/92 (51.1) |
| sCR/CR | 14 (9.0) | 11 (7.5) | 34 (22.3) | 31 (21.5) | 43 (30.4) | 44 (31.9) | 69 (45.4) | 62 (42.5) |
| sCR/CR + VGPR | 90 (58.4) | 83 (57.5) | 122 (80.3) | 107 (74.3) | 117 (83.0) | 111 (80.4) | 130 (85.5) | 121 (82.9) |
| PR or less to sCR/CR | – | – | 0/63 | 2/61 | 0/28 | 0/28 | – | – |
| VGPR to sCR/CR | – | – | 20/75 | 18/72 | 12/81 | 16/74 | – | – |
| Response . | After induction therapy . | After ASCT . | After consolidation . | Best response . | ||||
|---|---|---|---|---|---|---|---|---|
| Bor-HDM (n = 154) . | HDM (n = 146) . | Bor-HDM (n = 152*) . | HDM (n = 144) . | Bor-HDM (n = 141) . | HDM (n = 138) . | Bor-HDM (n = 152*) . | HDM (n = 146) . | |
| sCR | 9 (5.8) | 4 (2.7) | 16 (10.5) | 19 (13.2) | 27 (19.1) | 31 (22.5) | 45 (29.6) | 45 (30.8) |
| CR | 5 (3.2) | 7 (4.8) | 18 (11.8) | 12 (8.3) | 16 (11.3) | 13 (9.4) | 24 (15.8) | 17 (11.6) |
| VGPR | 76 (49.4) | 72 (49.3) | 88 (57.9) | 76 (52.8) | 74 (52.5) | 67 (48.6) | 61 (40.1) | 59 (40.4) |
| Partial response | 55 (35.7) | 54 (37.0) | 29 (19.1) | 31 (21.5) | 22 (15.6) | 22 (15.9) | 21 (13.8) | 20 (13.7) |
| Stable disease | 9 (5.8) | 9 (6.2) | 0 | 4 (2.8) | 0 | 3 (2.2) | 0 | 3 (2.1) |
| PD | 0 | 0 | 1 (0.7) | 2 (1.4) | 2 (1.4) | 2 (1.4) | 1 (0.7)† | 2 (1.4)† |
| Negative MRD‡ | 14/67 (20.9) | 15/67 (22.4) | 31/75 (41.3) | 26/66 (39.4) | 34/72 (47.2) | 32/67 (47.8) | 47/90 (52.2) | 47/92 (51.1) |
| sCR/CR | 14 (9.0) | 11 (7.5) | 34 (22.3) | 31 (21.5) | 43 (30.4) | 44 (31.9) | 69 (45.4) | 62 (42.5) |
| sCR/CR + VGPR | 90 (58.4) | 83 (57.5) | 122 (80.3) | 107 (74.3) | 117 (83.0) | 111 (80.4) | 130 (85.5) | 121 (82.9) |
| PR or less to sCR/CR | – | – | 0/63 | 2/61 | 0/28 | 0/28 | – | – |
| VGPR to sCR/CR | – | – | 20/75 | 18/72 | 12/81 | 16/74 | – | – |
Data are expressed as no. (%) or n/N (%) unless otherwise indicated. PD, progressive disease; PR, partial response.
One patient died of flu infection during transplant and 1 patient withdrew consent.
These 3 patients experienced disease progression after randomization and did not undergo HDT.
Sensitivity of 10−5.
Survival outcomes
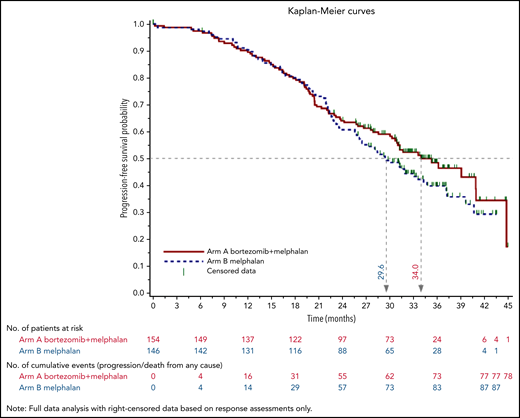
As of December 2018, median follow-up was 34.3 months (95% CI, 32.7-35.4) in arm A and 35.8 months (95% CI, 33.3-37.1) in arm B. Overall, 164 patients relapsed, with a median PFS of 34.0 months for Bor-HDM–treated patients and 29.6 months for HDM-treated patients (adjusted HR, 0.82; 95% CI, 0.61-1.13; P = .244) (Figure 4). Thirty patients died, with an estimated 3-year OS of 89.5% in both arms (adjusted HR, 1.28; 95% CI, 0.62-2.64; P = .374).
Engraftment and treatment-related toxicities
The number of CD34 cells collected was 9.19 × 106 cells/kg (first and third quartiles [Q1-Q3], 6.66-10.51) and 9.14 × 106 cells/kg (Q1-Q3, 6.40-10.42), respectively. Patients received at transplant a median of 3.45 × 106 (Q1-Q3, 2.73-4.70) and 3.38 × 106 (Q1-Q3, 2.70-5.00) CD34+ cells/kg, respectively. There was no engraftment failure or delay, and no difference between the 2 arms. Neutrophils (absolute neutrophil count ≥0.5 × 109/L) and platelets (≥20 × 109/L without transfusion) recovered in median times of 12.0 (Q1-Q3, 11.0-14.0) and 12.0 (Q1-Q3, 11.0-13.0) days. The median time to platelet level ≥50 × 109/L was 16.0 (Q1-Q3, 14.0-24.0) and 15.0 (Q1-Q3, 14.0-21.0) days. Patients were discharged from the transplant unit in median times of 19.0 (Q1-Q3, 17.0-21.0) and 18.0 (Q1-Q3, 16.0-20.0) days.
There were no deaths related to experimental conditioning regimen, but 1 patient died of flu-related acute respiratory distress syndrome at day 4 of transplantation. Data are summarized in Table 3. Concerning toxicities, 69 serious AEs occurred in 47 patients (18.7% of patients treated with Bor-HDM and 13.1% with HDM alone), including: 1 pulmonary embolism, 3 deep vein thrombosis, 2 cardiac failures, and 2 pericarditis; 1 acute respiratory distress syndrome, 4 pneumoniae, 1 acute pulmonary edema, and 1 cryptogenic organizing pneumonia; 3 hepatobiliary disorders; 2 erythema; 1 posterior reversible encephalopathy syndrome, 1 optic neuritis, and 3 PNY; 8 gastrointestinal tract disorders; and 17 infections and 2 secondary malignancies (1 fatal acute myeloid leukemia and 1 basal cell carcinoma).
Engraftment and transplant-related toxicities of interest
| Toxicities of interest . | Arm A, Bor-HDM . | Arm B, HDM . |
|---|---|---|
| Patients who underwent ASCT, n (%) | 151 (98) | 144 (98.5) |
| PBSC infused (106 CD34/kg), median (Q1-Q3) | 3.45 (2.73-4.71) | 3.38 (2.70-5.00) |
| Duration of hospital stay, median (Q1-Q3), d | 19 (17.0-21.0) | 18 (16.0-20.0) |
| Duration of neutropenia (ANC <0.5 × 109/L), median (Q1-Q3), d | 12 (11.0-14.0) | 12 (11.0-13.0) |
| Duration of thrombocytopenia | ||
| <20 × 109/L, median (Q1-Q3) | 12 d (11.0-14.0) | 12 d (11.0-13.0) |
| <50 × 109/L, median (Q1-Q3) | 16 d (14.0-24.0) | 15 d (14.0-21.0) |
| No. of platelet transfusions, median (Q1-Q3) | 2 (1-3) | 2 (1-3) |
| No. of packed red blood cell transfusions, median (Q1-Q3) | 0 (0-2) | 0 (0-2) |
| Patients with curative antibiotherapy, n (%) | 128 (84.8) | 122 (84.7) |
| Duration of curative antibiotherapy, median (Q1-Q3), d | 8.0 (6.0-12.5) | 9.0 (6.0-13.0) |
| Mucositis any grade, n (%) | 96 (64.0) | 95 (65.5) |
| Mucositis grade 3-4, n (%) | 38 (25.3) | 32 (22.1) |
| Duration of mucositis, median (Q1-Q3), d | 7.0 (5.0-10.0) | 8.0 (4.5-10.0) |
| Gastrointestinal tract/diarrhea, n (%) | 110 (73.3) | 105 (72.4) |
| Dermatologic/allergic reactions, n (%) | 19 (12.7) | 15 (10.3) |
| PNY, n (%) | 38 (25.3) | 15 (10.3) |
| Headache, n (%) | 33 (22.0) | 35 (24.1) |
| Death, n (%) | 1 (0.7) | 0 |
| Toxicities of interest . | Arm A, Bor-HDM . | Arm B, HDM . |
|---|---|---|
| Patients who underwent ASCT, n (%) | 151 (98) | 144 (98.5) |
| PBSC infused (106 CD34/kg), median (Q1-Q3) | 3.45 (2.73-4.71) | 3.38 (2.70-5.00) |
| Duration of hospital stay, median (Q1-Q3), d | 19 (17.0-21.0) | 18 (16.0-20.0) |
| Duration of neutropenia (ANC <0.5 × 109/L), median (Q1-Q3), d | 12 (11.0-14.0) | 12 (11.0-13.0) |
| Duration of thrombocytopenia | ||
| <20 × 109/L, median (Q1-Q3) | 12 d (11.0-14.0) | 12 d (11.0-13.0) |
| <50 × 109/L, median (Q1-Q3) | 16 d (14.0-24.0) | 15 d (14.0-21.0) |
| No. of platelet transfusions, median (Q1-Q3) | 2 (1-3) | 2 (1-3) |
| No. of packed red blood cell transfusions, median (Q1-Q3) | 0 (0-2) | 0 (0-2) |
| Patients with curative antibiotherapy, n (%) | 128 (84.8) | 122 (84.7) |
| Duration of curative antibiotherapy, median (Q1-Q3), d | 8.0 (6.0-12.5) | 9.0 (6.0-13.0) |
| Mucositis any grade, n (%) | 96 (64.0) | 95 (65.5) |
| Mucositis grade 3-4, n (%) | 38 (25.3) | 32 (22.1) |
| Duration of mucositis, median (Q1-Q3), d | 7.0 (5.0-10.0) | 8.0 (4.5-10.0) |
| Gastrointestinal tract/diarrhea, n (%) | 110 (73.3) | 105 (72.4) |
| Dermatologic/allergic reactions, n (%) | 19 (12.7) | 15 (10.3) |
| PNY, n (%) | 38 (25.3) | 15 (10.3) |
| Headache, n (%) | 33 (22.0) | 35 (24.1) |
| Death, n (%) | 1 (0.7) | 0 |
ANC, absolute neutrophil count; PBSC, peripheral blood stem cells.
Treatment-emergent AEs (TEAEs) were reported in almost all patients (96% in arm A vs 93.8% in arm B). There was no difference regarding TEAEs related to conditioning regimen (93.3% vs 89.7%). The proportion of grade 3/4 TEAEs was similar in both arms (54% vs 58.6%). Data of interest are shown in Table 3. During the whole program (including transplantation and consolidation), the most common (>10%) grade 3/4 AEs according to system organ class were blood and lymphatic system disorders (28.0% vs 33.8%), gastrointestinal disorders (20.0% vs 22.8%), general disorders (18.0% vs 11.0%), and infections (12.0% vs 15.2%). Overall, 46% of the patients in the Bor-HDM arm developed treatment-emergent PNY (all grade) vs 29.7% in the control arm. Grade 3/4 PNY or grade 2 neuralgia was reported in 11 patients (4% vs 3.4%). It should be noted that PNY was present at time of randomization in 78 (26%) patients.
Discussion
HDM (200 mg/m2) is the standard conditioning regimen before transplantation in patients with MM for >30 years. The IFM and other groups have shown, in large phase 2 to 3 trials, the superiority of frontline transplantation even in the era of RVd or carfilzomib, lenalidomide and dexamethasone (KRd) induction, consolidation, and lenalidomide-based maintenance regimens.19,20 Indeed, transplantation can deepen and prolong responses. However, transplant programs are thus far not curative, and most patients will relapse within 5 to 8 years. Several approaches have been evaluated for a long-time to enhance the efficacy of transplantation. These have included the use of a higher dose of melphalan33 and the use of total body irradiation,21,22 as well as the incorporation of other drugs into the conditioning regimen.23-25 These approaches usually resulted in increased morbidity and mortality without improvement in efficacy or outcomes. Over the last 10 years, several studies have provided evidence for potential synergism and safety when combining bortezomib with HDM. Consistent with reports by Lonial et al,34 and others,35-37 we previously showed promising results of Bor-HDM treatment in a phase 2 study, regardless of induction therapy.30
The aim of the present randomized phase 3 trial was to determine whether the Bor-HDM combination would confirm higher CR rates after transplant vs HDM alone, in the era of bortezomib-based triplet induction and consolidation regimen (before daratumumab), without the burden of increased toxicity. This randomized phase 3 trial enrolled 300 patients at 47 IFM transplant centers in 1.5 years. It did not confirm the results reported in our phase 2 study: the Bor-HDM conditioning regimen did not increase response rates posttransplant. On an intention-to-treat analysis, there was no difference regarding sCR/CR rates at day 60: 22.1% in the Bor-HDM arm vs 20.5% in the HDM arm (P = .844). One could argue that these sCR/CR rates are lower than expected, that we may have overestimated the CR rates to calculate our sample size, and therefore that we did not have sufficient statistical power to detect a difference between the 2 study arms. Nevertheless, because the 2-point difference in response was not clinically significant, we were confident that the lack of statistical significance was mainly related to the lack of difference rather than to a lack of statistical power. A confounding variable for outcomes could be the early read-out of results (day 60 instead of classical day 100), and we know that hematologic response can be completed within 3 to 6 months after transplantation. We therefore analyzed response rates at the completion of consolidation (estimated day 120 after transplantation). It was planned that all patients were to receive within the program two VTd cycles as consolidation, and 258 of 300 patients received at least 2 cycles. Only a few patients according to their treating physician received additional VTd consolidation cycles (n = 5), or lenalidomide and dexamethasone consolidation (n = 16), but all patients were analyzed at the completion of 2 cycles. There was again no statistical difference in terms of sCR/CR rates at the completion of consolidation (30.4% vs 31.9%; P = .626) and no difference in best response achieved all along during the program (45.4% vs 42.4% achieved sCR/CR, respectively). A difference could possibly occur at a deeper level, and MRD evaluation could be a key point. With a sensitivity of 10−5, ∼40% of patients were MRD undetectable according to MFC after transplant, and rates were comparable between the 2 arms (41.3% vs 39.4%; P = .864). There was no difference either at the completion of consolidation (47.2% vs 47.8%) or at any time during the program. Considering the poor chance of having a statistically significant difference, we did not perform MRD assessment by next-generation sequencing (sensitivity of 10−6), but marrow samples were stored. Finally, the absence of difference could be related either to a variable melphalan exposure when using fixed-dose melphalan or to previous bortezomib exposure. It is therefore possible that patients who had a suboptimal response to induction may be the most likely to benefit from the synergistic effects of bortezomib and alkylating agents. All patients in this trial received a bortezomib-based induction (mainly VTd or CyBorD) compared with 33% in our previous phase 2 study. Induction regimens were well balanced between the 2 arms, and randomization was stratified according to response; most of the patients already achieved a very good quality of response after induction, and >20% were MRD undetectable. Consequently, no difference could be seen according to induction regimen, and we cannot speculate that patients who had a suboptimal response to induction may be the most likely to benefit from the addition of bortezomib.
One could also argue that responses may be related to initial risk status of the disease, and trials have suggested that transplant could mainly benefit high-risk patients, with sustained responses and prolonged survivals.32 Promising data have been reported in studies with intravenous busulfan/melphalan.38-42 Although there was no difference in CR rates or better at day 100 (26% vs 34%) after busulfan/melphalan and transplantation, Qazilbash’s group (MD Anderson Cancer Center) (Bashir et al,41 Saini et al42) reported in their phase 3 study, with a median follow-up of 28 months, a statistically significant difference regarding PFS: not achieved in the busulfan + HDM arm vs 31 months in the HDM arm (P = .013), mainly in high-risk patients.41,42 In our study, after a median follow-up of 35 months’ postrandomization, there was still no PFS difference between the 2 arms, with a median PFS of 34.0 months with Bor-HDM. Thus, this longer follow-up did not reveal a PFS advantage; the study was not powered to that purpose, however. We evaluated outcomes according to risk status (data not shown), and the interaction test was not statistically significant to conclude to a different treatment effect in the high-risk subgroup. The absence of maintenance therapy could have been another confounding variable resulting in a lack of response. Some patients, according to their treating physician, may have received lenalidomide maintenance. We do not have these data but, because lenalidomide maintenance was not reimbursed at that time in France, it may concern only a small proportion of patients.
In terms of safety, this study confirmed that the addition of bortezomib to HDM conditioning did not significantly increase toxicity or delay engraftment. Twenty-eight patients had serious AEs related to the bortezomib conditioning vs 19 patients in the HDM arm. As expected, there was an excess of neurologic side effects in the Bor-HDM–treated population, and, overall, 70 patients in the Bor-HDM arm developed treatment-emergent PNY vs 38 patients in the control arm. Only a few patients could not receive the planned VTd consolidation, and there was no significant difference between the 2 arms. There was no difference regarding AEs of interest: grade 2 or higher neuropathy, skin rash, mucositis, diarrhea or constipation, cardiac rhythm disorders, or thromboembolic events.
In summary, in this phase 3 randomized, multicenter, open-label trial, 151 (98%) of 154 patients received the planned conditioning regimen with bortezomib and HDM. Although we confirm its safety with no engraftment delays or unexpected toxicities reported, the addition of bortezomib to HDM as a conditioning regimen pre-ASCT failed to show superiority over HDM alone. There was no significant difference in sCR/CR rates at day 60 posttransplant between the 2 arms or after consolidation therapy. Undetectable MRD rates were similar at any time point. With an extended follow-up, there was no PFS or OS advantage, even for high-risk patients. Although recent data with a busulfan-containing preparative regimen were favorable in high-risk patients, we believe that, to date, HDM (200 mg/m2) remains the standard-of-care conditioning regimen for intensive therapy with ASCT in MM. An interesting approach could be the addition of a more potent proteasome inhibitor instead of bortezomib, and a carfilzomib/melphalan preparative regimen has been evaluated in relapsed patients with a promising efficacy and safety signal.43
Acknowledgments
The authors gratefully acknowledge the work performed by the transplant unit and the clinical research unit in Toulouse, the IFM head office, and all the IFM participating centers, especially the transplant teams, and the individual research teams at all study sites. They especially acknowledge S. Rollet, L. Devlamynck, C. Payen, A. Huynh, S. Guenounou, F. Marguerite, P. Laroche, S. Degeilh, A. Huguet, M.E. Llau, S. Lodin, C. Chevalier, C. Mathiot, and M.O. Petillon; and they are also indebted to S. Gibbs (Eastern Health-Box Hill) for his critical reading of the manuscript. The authors thank all the members of the IFM for its participation in this study.
This trial was supported by research funding from Janssen-Cilag and the French government (PHRC-K).
Authorship
Contribution: M.A. and M.R. designed the study; M.R., M.M., X.L., B.R., C.H., L.K., A.P., C.T., M.-L.C., S.R., M.D., E.N.-V., M.E.-B., K.B., C.M., A.-M.S., C.A., C.D., J.F., B.K., L.G., S.B., J.-V.M., A.J., P.L., C.B., B.H., O.B., V.D., S.M., K.A.-M., M.-C.V., E.R., D.C., J. Caers, C.C., L.B., L.V., S.G., P.Z., B.S., J.-R.E., C.H.-K., V.M., P. Mineur, J.-C.E., H.D., V.R., M.V., D.C., T.F., P. Moreau, and M.A. recruited subjects for the study; S.W. performed the MRD analysis by MFC; H.A.-L. and J. Corre performed the cytogenetics analysis; A.-L.C. and P.O. performed the pharmacovigilance; C.P. reviewed responses; V.L.-C. and L.D. analyzed the data and performed the statistical analyses; M.R., V.L.-C., and M.A. wrote the paper; and all authors were involved in analyzing and interpreting the data and checked the final version of the manuscript; the authors were fully responsible for content and editorial decisions for the manuscript.
Conflict-of-interest disclosure: M.R., M.M., X.L., B.R., C.H., L.K., A.P., C.T., M.-L.C., M.D., K.B., A.-M.S., C.D., B.K., L.G., A.J., B.H., V.D., S.M., M.-C.V., D.C., J. Caers, C.C., L.B., L.V., P.Z., B.S., J.-R.E., H.D., V.R., M.V., D.C., T.F., P. Moreau, H.A.-L., J. Caers, and M.A. participated in lectures and advisory boards for Janssen. The remaining authors declare no competing financial interests.
A complete list of the members of the Intergroupe Francophone du Myélome (IFM) appears in the supplemental appendix.
Correspondence: Murielle Roussel, Service d’Hématologie Clinique, Centre Hospitalo-universitaire (CHU) Dupuytren, Limoges, France; e-mail: murielle.roussel@chu-limoges.fr.
Initial results were reported during an oral session at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-12 December 2017 (abstract #398).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal