Key Points
IL-7R immunotherapy with LUSV shows in vivo efficacy in BCP- and T-ALL xenografts, especially when combined with polychemotherapy.
LUSV blocks IL-7R signaling and induces ADCP in ALL PDX cells, which correlates with IL-7R surface expression.
Visual Abstract
Acute lymphoblastic leukemia (ALL) arises from the uncontrolled proliferation of B-cell precursors (BCP-ALL) or T cells (T-ALL). Current treatment protocols obtain high cure rates in children but are based on toxic polychemotherapy. Novel therapies are urgently needed, especially in relapsed/refractory (R/R) disease, high-risk (HR) leukemias and T-ALL, in which immunotherapy approaches remain scarce. Although the interleukin-7 receptor (IL-7R) plays a pivotal role in ALL development, no IL-7R–targeting immunotherapy has yet reached clinical application in ALL. The IL-7Rα chain (CD127)–targeting IgG4 antibody lusvertikimab (LUSV; formerly OSE-127) is a full antagonist of the IL-7R pathway, showing a good safety profile in healthy volunteers. Here, we show that ∼85% of ALL cases express surface CD127. We demonstrate significant in vivo efficacy of LUSV immunotherapy in a heterogeneous cohort of BCP- and T-ALL patient-derived xenografts (PDX) in minimal residual disease (MRD) and overt leukemia models, including R/R and HR leukemias. Importantly, LUSV was particularly effective when combined with polychemotherapy in a phase 2-like PDX study with CD127high samples leading to MRD-negativity in >50% of mice treated with combination therapy. Mechanistically, LUSV targeted ALL cells via a dual mode of action comprising direct IL-7R antagonistic activity and induction of macrophage-mediated antibody-dependent cellular phagocytosis (ADCP). LUSV–mediated in vitro ADCP levels significantly correlated with CD127 expression levels and the reduction of leukemia burden upon treatment of PDX animals in vivo. Altogether, through its dual mode of action and good safety profile, LUSV may represent a novel immunotherapy option for any CD127+ ALL, particularly in combination with standard-of-care polychemotherapy.
Introduction
Acute lymphoblastic leukemia (ALL) is the most frequent childhood cancer and arises from the uncontrolled proliferation of B-cell precursor or T cells (BCP- or T-ALL). Current treatment protocols achieve cure rates of up to 90% in children, yet they are based on toxic polychemotherapy.1 Furthermore, some subgroups such as KMT2A-rearranged (KMT2A-r), BCR::ABL1+ and BCR::ABL1–like BCP-ALL as well as high-risk (HR) T-ALL and adult ALL are still associated with adverse outcomes.1,2 Novel immunotherapy options are urgently needed to substitute unspecific chemotherapy and improve survival after relapse.
One promising novel target is the interleukin-7-receptor (IL-7R). IL-7R is composed of 2 subunits, the IL-7Rα chain (CD127) and the common γ-chain (CD132, γc, IL2RG), which, upon IL-7 binding, form a heterodimer and induce proliferative and antiapoptotic downstream signaling mainly via JAK1/3-STAT5 but also via MAPK/ERK and PI3K/AKT/mTOR (reviewed by Barata et al3). CD127 is a component of the thymic stromal lymphopoietin (TSLP) receptor and required for TSLP signaling.3 IL-7R signaling is crucial for the development and homeostasis of functional B and T cells in mice and humans.3 Recent data show involvement of the IL-7R pathway in the development and progression of BCP- and T-ALL.4-8 This may occur by gain of function (GoF) mutations, which predominantly occur in exon 6 and are present in a small fraction of BCP-ALLs and ∼10% of T-ALL cases.9 Such GoF mutations can initiate a preleukemic stage in B-cell precursors and promote T-ALL alone or in conjunction with MYC.4,5,8 On the contrary, overexpression of nonmutational CD127 was sufficient to induce malignant transformation and IL-7 independence in T-ALL.6 Other mutational events, for example, NOTCH1 and RAS alterations, may fuel IL-7R–mediated tumor progression.10,11
We and others had previously shown that CD127 can be targeted using research-grade immunoglobulin G1 (IgG1) monoclonal antibodies (mAbs) leading to significantly reduced leukemic burden in patient–derived xenograft (PDX) models of BCP- and T-ALL.12-14 However, no clinically available IL-7R immunotherapy has to date been translated into ALL treatment.
The IL-7R antibody lusvertikimab (LUSV; formerly OSE-127) is a humanized IgG4 mAb that, through its binding to site-1 and site-2b of CD127, prevents the heterodimerization and subsequent activation of the IL-7R, without affecting TSLP receptor signaling.15 LUSV differs from previously developed anti–IL-7R mAbs by its absence of antibody-induced receptor internalization, its lack of antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxic activity, and absence of agonistic activity on IL-7/IL-7R signaling.13,14,16-18 LUSV administration demonstrated an excellent safety profile during a phase 1 clinical trial (NCT03980080) conducted in 63 healthy volunteers. No cytokine release, nor significant lymphopenia or peripheral T-cell subtype modifications were observed.18 Based on its safety profile and IL-7R antagonistic properties, LUSV is currently evaluated in phase 2 trials in ulcerative colitis (CoTikiS study: NCT04882007) and Sjögren syndrome (NCT04605978). The aim of this study was to evaluate the preclinical efficacy of LUSV in ALL.
We show that CD127 is expressed in the vast majority of ALL cases including T-ALL and HR-cytogenetic BCP-ALL subgroups. Using minimal residual disease (MRD)-like, postchemotherapy MRD, and overt leukemia PDX models, we observed profound preclinical in vivo efficacy of LUSV monotherapy and enhanced potency when combined with chemotherapy. Mechanistically, we found that LUSV blocked prosurvival and proliferative IL-7R signaling in IL-7 responsive ALLs and induced antibody-dependent cellular phagocytosis (ADCP) of leukemic cells in correlation with CD127 surface expression levels irrespective of IL-7R pathway activity.
Methods
Flow cytometry
Prospective measurements of diagnostic ALL patient samples were conducted in accordance with EuroFlow standards.19,20 Whole blood or bone marrow (BM) samples were washed twice in phosphate-buffered saline/0.09% NaN3/0.2% bovine serum albumin. For surface staining, samples (maximum 5 × 106 cells in 50 μL) were incubated with an antibody cocktail (CD45-APC-H7 clone 2D1; CD19-PE-Cy7 clone J3-119; and CD127-BV421 clone A019D5) at room temperature for 15 minutes. Measurements were performed on a BD FACSLyricTM, and 100 000 events were acquired when possible.
Xenograft experiments
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NOD scid gamma [NSG]) mice were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were bred under pathogen-free conditions and xenografts were generated in accordance with governmental regulations (Schleswig-Holstein Ministerium für Landwirtschaft, ländliche Räume, Europa und Verbraucherschutz) as described previously.12,21-26 PDX cells were injected IV into female NSG mice (age, 6-10 weeks), and leukemic engraftment was followed by detection of human (h)CD45+/murine (m)CD45–/hCD19+ cells in the peripheral blood (PB) for BCP-ALL and by detection of hCD45+/mCD45–/hCD7+ cells for T-ALL via flow cytometry analysis. Antibody treatment was conducted on day +1, +3, +7, and +14 and every 14 days thereafter as described previously.12,22 Induction-like polychemotherapy (vincristine 10 μg IV, day 1; dexamethasone 70 μg intraperitoneally, day 1-5; pegylated (PEG)-asparaginase 100 IU, IV, day 1) was conducted as described previously.23,25,27 MRD in BCP-ALL and T-ALL PDX mice was measured in BM samples by quantitative polymerase chain reaction or digital droplet polymerase chain reaction for patient–specific Ig/B-cell receptor or T-cell receptor gene rearrangements, respectively.23,25 Further information is available in supplemental Methods, available on the Blood website.
Antibodies
Therapeutic anti-CD127 antibodies were generated by OSE Immunotherapeutics and found free from endotoxin.
Phagocytosis assays
Patients with leukemia were treated according to AIEOP-BFM ALL 2000 or 2009 protocols or COALL after informed consent in accordance with the Declaration of Helsinki. Prospective measurements of CD127 were conducted in patients included in the AIEOP-BFM ALL 2017 or EsPhALL 2017 study after informed consent. The study was approved by the ethical committee of the Christian-Albrecht University Kiel (D437/17). Leukemia-xenografts were generated in accordance with local governmental regulations (Schleswig-Holstein Ministerium für Landwirtschaft, ländliche Räume, Europa und Verbraucherschutz).
Results
CD127 is expressed in the majority of BCP-ALL and T-ALL cases
To identify patients who may potentially benefit from CD127 immunotherapy, CD127 surface expression was prospectively measured via flow cytometry in 371 diagnostic samples of patients with BCP- and T-ALL.19,20
We detected CD127-positivity (defined as ≥10% CD127+ ALL cells by flow cytometry21; gating strategy depicted in supplemental Figure 1A) in 313 of 371 cases (84.4%), of which 147 (39.6%) were CD127hi (≥50% CD127+ blasts; Figure 1A). CD127 surface expression was mostly unimodal and rarely bimodal (∼90% vs ∼10%; supplemental Figure 1B). The analysis of ALL subgroups exposed a higher number of CD127hi cases in T-ALL than in BCP-ALL (54.3% vs 38.1%) and higher frequencies of CD127+ cells in T-ALL than BCP-ALL cases (average 55.8% vs 40.5% CD127+ cells; P = .0106; median mean fluorescence intensity (MFI), 494 vs 356; Figure 1B-D; supplemental Figure 1C). Among BCP-ALL cases, particularly high expression levels were detected in TCF3::PBX1+ ALL (69.1% CD127+ cells; median MFI, 663.0; Figure 1E; supplemental Figure 1C). Furthermore, CRLF2-rearranged BCP-ALL cases exposed high CD127 expression levels (53.0% CD127+ cells; median MFI, 458.0; Figure 1E; supplemental Figure 1C) and patients with BCP-ALL with an IKZF1+ signature28 (56.0% CD127+ cells; median MFI, 554.0). Moreover, 4 of 4 TP53–mutated BCP-ALL cases were CD127hi (average 73.65% CD127+ cells; median MFI, 1028.0; Figure 1E; supplemental Figure 1C). Particularly low CD127 expression was detected in ZNF384-fusion–positive BCP-ALL cases (average 8.0% CD127+ cells; median MFI, 183.5; Figure 1E; supplemental Figure 1C).
CD127 is expressed in the majority of BCP-ALL and T-ALL cases. CD127 surface expression was prospectively measured via flow cytometry in 371 diagnostic blood or BM samples of patients with BCP-ALL and T-ALL in accordance with International-BFM-FLOW recommendations.19,20 CD127 low positivity was defined as ≥10% CD127+ ALL cells by flow cytometry and high expression as ≥50% CD127+ blasts within the CD45dim/CD19+ (BCP-ALL) or CD45dim/CD7+ (T-ALL) cell population, respectively. (A-C) Pie charts depicting the ratio of patients with CD127-negative, CD127-low, and CD127-high ALL among all analyzed patient samples (A), BCP-ALL (B), and T-ALL cases only within the cohort (C). (D) Comparison of percentage of CD127+ ALL cells in BCP-ALL vs T-ALL cases; ∗∗P = .0023, unpaired 2-sided t test. (E) Ratio of CD127+ ALL cells in different BCP-ALL subgroups. The stratification relevant lesions ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, and KMT2A-rearrangements (KMT2A-r) were diagnosed by fluorescence in situ hybridization or RNA sequencing. Further genetic alterations (CRLF2-rearrangements [CRLF2-r], TP53-mutations [TP53-mut], and ZNF384-fusions [ZNF384]) were detected within the “B-others” subgroup (BCP-ALL cases without ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, and KMT2A-r). Blue lines show the cutoffs for low and high CD127-positivity. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
CD127 is expressed in the majority of BCP-ALL and T-ALL cases. CD127 surface expression was prospectively measured via flow cytometry in 371 diagnostic blood or BM samples of patients with BCP-ALL and T-ALL in accordance with International-BFM-FLOW recommendations.19,20 CD127 low positivity was defined as ≥10% CD127+ ALL cells by flow cytometry and high expression as ≥50% CD127+ blasts within the CD45dim/CD19+ (BCP-ALL) or CD45dim/CD7+ (T-ALL) cell population, respectively. (A-C) Pie charts depicting the ratio of patients with CD127-negative, CD127-low, and CD127-high ALL among all analyzed patient samples (A), BCP-ALL (B), and T-ALL cases only within the cohort (C). (D) Comparison of percentage of CD127+ ALL cells in BCP-ALL vs T-ALL cases; ∗∗P = .0023, unpaired 2-sided t test. (E) Ratio of CD127+ ALL cells in different BCP-ALL subgroups. The stratification relevant lesions ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, and KMT2A-rearrangements (KMT2A-r) were diagnosed by fluorescence in situ hybridization or RNA sequencing. Further genetic alterations (CRLF2-rearrangements [CRLF2-r], TP53-mutations [TP53-mut], and ZNF384-fusions [ZNF384]) were detected within the “B-others” subgroup (BCP-ALL cases without ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, and KMT2A-r). Blue lines show the cutoffs for low and high CD127-positivity. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
These data indicate that a substantial proportion of ALL cases, including patients with unfavorable cytogenetics, could be considered for CD127 immunotherapy.
LUSV reduces leukemic engraftment in MRD-like in vivo models of ALL
Due to the high expression of CD127 in TCF3::PBX1+ BCP-ALL cases, we tested the in vivo efficacy of LUSV in 2 TCF3::PBX1+ PDX models (with 65% and 83.4% CD127+ cells; supplemental Table 1, BCP-ALL PDX number 1-2). An MRD-like model was applied, starting therapy 1 day after PDX-cell injection (supplemental Figure 2A).22,23,25,27 LUSV therapy led to a significant decrease in PB blasts (PBBs) in both models (median PBBs, 27.51% vs 1.55%; P < .0001; and 29.23% vs 0.86%; P = .0002, respectively; Figure 2A), which translated into a survival prolongation of LUSV-treated mice (median, 82 days vs not reached; P < .0001; and 91 days vs not reached; P < .0001, respectively; Figure 2B). Upon termination of the experiment at 160 days, no LUSV-treated animal had developed clinical signs of leukemia, and 10 of 10 and 8 of 10 treatment mice, respectively, were MRD negative (Figure 2C).
LUSV reduces leukemic burden in MRD in vivo models of BCP-ALL and T-ALL. (A-C) Immunodeficient mice were transplanted with PDX cells from 2 different TCF3::PBX1+ patients (63.0% and 89.6% CD127+ blasts, respectively) and treated with LUSV (5 mg/kg) or a control vehicle (n = 10, respectively) starting the day after injection, modeling an MRD-like situation (IV treatment on day +1, +3, +7, and +14 and every 14 days thereafter as described previously12). (A) PB of both, control and LUSV-treated animals was withdrawn when the first PDX mouse showed signs of overt leukemia and compared for the ratio of hCD45+/hCD19+/mCD45– cells in the PB as measured via flow cytometry, unpaired 2-sided t test. Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. (B) Therapy-associated differences in the survival of NSG mice were determined using Kaplan-Meier log-rank statistics. (C) The experiment was terminated after 170 days, and BM samples of euthanized animals were analyzed for MRD by PCR for patient–specific Ig/T-cell receptor rearrangements. Overt ALL = mouse showed clinical signs of leukemia upon euthanization; neg, negative (below detection limit). (D-F) A phase 2-like PDX study was performed using CD127-high (≥50% CD127+ cells) T-ALL PDX samples (n = 8 patients) including 3 samples from R/R disease. Two NSG mice per patient were injected with PDX cells, randomly assigned into treatment groups and LUSV therapy was conducted in an MRD-like setting. (D) Experimental setup and therapy scheme. (E) Blood of both, control and LUSV-treated animals bearing the same PDX sample was withdrawn when one of the 2 PDX mice showed signs of overt leukemia and the number of hCD45+/hCD19+/mCD45– cells in the PB was measured via flow cytometry. The waterfall plot shows the difference in PBBs between respective control and LUSV-treated mice (sorted from weakest therapy response to highest therapy response). Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. (F) Therapy-associated differences in the survival of NSG mice was determined using Kaplan-Meier log-rank statistics. (G-H) Cells from 1 T-ALL PDX (T-ALL PDX number 1) were injected into replicate mice (n = 3 for no-treatment group [control]; n = 5 for treatment groups), animals were subjected to chemotherapy upon 10% PBBs and LUSV immunotherapy (chemotherapy + LUSV) vs mock treatment (chemotherapy only) was initiated upon reoccurrence of PBB (termed “postchemo-MRD” model).23 (G) Schematic depiction of the experimental setup of the postchemotherapy MRD model. (H) Therapy-associated differences in the survival of NSG mice was determined using Kaplan-Meier log-rank statistics. One mouse in the chemotherapy only group died due to procedural complications. The experiment was terminated after 130 days. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. n.r., not reached.
LUSV reduces leukemic burden in MRD in vivo models of BCP-ALL and T-ALL. (A-C) Immunodeficient mice were transplanted with PDX cells from 2 different TCF3::PBX1+ patients (63.0% and 89.6% CD127+ blasts, respectively) and treated with LUSV (5 mg/kg) or a control vehicle (n = 10, respectively) starting the day after injection, modeling an MRD-like situation (IV treatment on day +1, +3, +7, and +14 and every 14 days thereafter as described previously12). (A) PB of both, control and LUSV-treated animals was withdrawn when the first PDX mouse showed signs of overt leukemia and compared for the ratio of hCD45+/hCD19+/mCD45– cells in the PB as measured via flow cytometry, unpaired 2-sided t test. Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. (B) Therapy-associated differences in the survival of NSG mice were determined using Kaplan-Meier log-rank statistics. (C) The experiment was terminated after 170 days, and BM samples of euthanized animals were analyzed for MRD by PCR for patient–specific Ig/T-cell receptor rearrangements. Overt ALL = mouse showed clinical signs of leukemia upon euthanization; neg, negative (below detection limit). (D-F) A phase 2-like PDX study was performed using CD127-high (≥50% CD127+ cells) T-ALL PDX samples (n = 8 patients) including 3 samples from R/R disease. Two NSG mice per patient were injected with PDX cells, randomly assigned into treatment groups and LUSV therapy was conducted in an MRD-like setting. (D) Experimental setup and therapy scheme. (E) Blood of both, control and LUSV-treated animals bearing the same PDX sample was withdrawn when one of the 2 PDX mice showed signs of overt leukemia and the number of hCD45+/hCD19+/mCD45– cells in the PB was measured via flow cytometry. The waterfall plot shows the difference in PBBs between respective control and LUSV-treated mice (sorted from weakest therapy response to highest therapy response). Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. (F) Therapy-associated differences in the survival of NSG mice was determined using Kaplan-Meier log-rank statistics. (G-H) Cells from 1 T-ALL PDX (T-ALL PDX number 1) were injected into replicate mice (n = 3 for no-treatment group [control]; n = 5 for treatment groups), animals were subjected to chemotherapy upon 10% PBBs and LUSV immunotherapy (chemotherapy + LUSV) vs mock treatment (chemotherapy only) was initiated upon reoccurrence of PBB (termed “postchemo-MRD” model).23 (G) Schematic depiction of the experimental setup of the postchemotherapy MRD model. (H) Therapy-associated differences in the survival of NSG mice was determined using Kaplan-Meier log-rank statistics. One mouse in the chemotherapy only group died due to procedural complications. The experiment was terminated after 130 days. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. n.r., not reached.
Based on the pronounced effect of LUSV in CD127hi BCP-ALL samples, we next tested the efficacy of LUSV in T-ALL, conducting a preclinical phase 2-like PDX study.22,23,25,27,30 Two mice per patient were injected with CD127hi T-ALL PDX samples including 3 samples from patients with relapsed/refractory (R/R) T-ALL (supplemental Table 1, T-ALL PDX number 1-8; Figure 2D) and randomly assigned into treatment groups. Again, an MRD-like setting was applied and when a mouse (control or LUSV-treated) developed clinical signs of overt leukemia, PB samples of both control and LUSV–treated PDX mice were analyzed for leukemic cells in the PB, and individual mouse survival was followed until reaching abortion criteria22,27 (Figure 2D). We observed an in vivo response in 8 of 8 (100%) LUSV–treated PDX samples (ranging from 9% to 92% PBB reduction [ΔPBB = PBBControl-treated – PBBLUSV-treated] between control and corresponding LUSV–treated PDX animals; Figure 2E), resulting in a survival prolongation of LUSV–treated mice (median, 124 days vs 75.5 days for controls; P = .0048; Figure 2F). Overall, 3 of 8 (37.5%) PDX mice in the treatment group, including 1 mouse bearing PDX cells obtained from a patient with R/R, survived and were MRD-negative upon termination of the experiment (supplemental Figure 2B-C). We next mimicked a further clinical situation, examining sequential treatment of chemotherapy and antibody therapy in vivo (termed “postchemo-MRD”).23 PDX cells from T-ALL PDX number 1 were injected into replicate mice, animals were subjected to chemotherapy when 10% blasts were detected in the PB, and immunotherapy was initiated upon recurrence of PBB (Figure 2G). LUSV was highly efficient in this setting, leading to a survival prolongation compared with chemotherapy only (median, 87 days in chemotherapy only vs not reached in postchemotherapy LUSV). Three of 5 animals survived the experimental period and were MRD-low positive or MRD negative upon euthanasia (Figure 2H; supplemental Figure 2D).
Taken together, these results suggest that LUSV is effective in PDX models of BCP- and T-ALL MRD and postchemotherapy MRD, including R/R T-ALL cases.
LUSV is effective in overt leukemia PDX models, including R/R ALL
Next, we tested the efficacy of LUSV modeling overt ALL (supplemental Figure 3A-B). We conducted a preclinical phase 2-like PDX study12,23 using 24 PDX samples from 15 patients with BCP-ALL and 9 with T-ALL including BCP-ALL PDX samples representing HR-ALL profiles such as 3 BCR::ABL1+, 2 KMT2A-r, and 3 “B-other” PDX samples (Table 1). This cohort contained 7 PDX samples of R/R disease (1 × BCP-ALL PDX and 6 × T-ALL PDX; Table 1).23 To model overt leukemia, therapy was initiated when mice showed 1% blasts in the PB, and comparative PB analysis was conducted when 1 of the 2 PDX mice (control vs LUSV-treated) showed clinical signs of leukemia.22,23 A blast reduction was observed in 23 of 24 (96%) LUSV–treated BCP-ALL and T-ALL PDX animals (ΔPBB, 18.0%-88.8%; Figure 3A; Table 1). Accordingly, LUSV-treated animals showed a survival prolongation compared with the control group (median survival, 82.5 days vs 58.5 days; P = .0030; Figure 3B). Furthermore, in vivo response was detected in all R/R PDX samples (Figure 3A-B; Table 1). Particularly favorable responses to LUSV therapy were observed in TCF3::PBX1+ PDX samples, including 1 matched pair of initial diagnosis and relapse (BCP-ALL PDX number 4-5; ΔPBB = 52.8% and 74.7%, respectively). Pronounced LUSV efficacy was observed in 2 “B-other” PDX samples: 1 bearing a DNMT3B::PAX5 fusion31 and an IL-7R GoF mutation (ΔPBB = 73.2% BCP-ALL PDX number 12) and 1 with a P2RY8::CRLF2 fusion (ΔPBB = 81.5% BCP-ALL PDX number 15) that was MRD negative upon end of the experiment (supplemental Figure 3C). Moreover, among the best LUSV responders was a de novo T-ALL sample carrying a STAT5 mutation (ΔPBB = 88.8% T-ALL PDX number 1). Of note, the intensity of the in vivo response to LUSV correlated with the frequency of CD127+ cells, as determined via flow cytometry (Pearson r = 0.6924; P = .0002; Figure 3C).
Clinical characteristics of BCP-ALL and T-ALL PDX samples used in BCP-ALL/T-ALL phase 2-like overt leukemia study (refers toFigure 3)
| Patient characteristics . | PDX data . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDX no. . | Age at diagnosis, y . | Sex . | White blood cell count/µl . | Relapse . | Death . | Immunophenotype . | Cytogenetics∗ . | Relevant mutations† . | Sample origin . | CD127+ cells,‡ % . | Delta PBB§ . | Survival control‖ . | Survival LUSV¶ . |
| BCP-ALL 1 | 10.4 | M | 7.8 | No | No | Common B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 65 | 68.42 | 84 | 129 |
| BCP-ALL 2 | 14.8 | M | 26.8 | No | No | Pre-B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 83.4 | 63.25 | 99 | 227 |
| BCP-ALL 3 | 8.6 | F | 12.6 | No | No | Common B-ALL | TCF3::PBX1 | NRAS (p.G13D) | De novo BCP-ALL | 45.9 | 25.9 | 59 | 60 |
| BCP-ALL 4∗∗ | 13.4 | M | 12.32 | Yes | Yes | Pre-B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 67.7 | 52.79 | 56 | 85 |
| BCP-ALL 5∗∗ | 13.4 | M | 12.32 | Yes | Yes | Pre–B-ALL | TCF3::PBX1 | n. d. | R/R BCP-ALL | 77.7 | 74.4 | 65 | 111 |
| BCP-ALL 6 | 17.3 | F | 13.3 | Yes | No | Common B-ALL | BCR::ABL1 | n. d. | De novo BCP-ALL | 65.5 | 68.4 | 58 | 135 |
| BCP-ALL 7 | 9.8 | F | 132 | No | No | Common B-ALL | BCR::ABL1 | n. d. | De novo BCP-ALL | 38.2 | 46 | 61 | 62 |
| BCP-ALL 8 | 9 | M | 3.5 | No | No | Common B-ALL | BCR::ABL1 | n. d. | De novo BCP-ALL | 59.4 | 51.6 | 84 | 119 |
| BCP-ALL 9 | 0.7 | F | 103 | No | No | Pre–B-ALL | KMT2A::MLLT1 | n. d. | De novo BCP-ALL | 45.1 | 24.9 | 55 | 62 |
| BCP-ALL 10 | 1.2 | M | 60.8 | Yes | Yes | Common B-ALL | KMT2A::MLLT10 | n. d. | De novo BCP-ALL | 29.8 | 30.9 | 37 | 44 |
| BCP-ALL 11 | 1.6 | M | 288.4 | No | No | B-ALL | B-other# (EBF1::PDGFRB) | n. d. | De novo BCP-ALL | 37.5 | 2 | 88 | 97 |
| BCP-ALL 12 | 3.1 | F | 103.53 | Yes | No | Common B-ALL | B-other (DNMT3B::PAX5) | IL7R (p.L243_T244delinsRCGI) (GoF) | De novo BCP-ALL | 56.6 | 73.18 | 83 | 228 |
| BCP-ALL 13 | 10.7 | M | 113 | No | No | Common B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 86.8 | 56.11 | 77 | 100 |
| BCP-ALL 14 | 5 | M | 204 | Yes | Yes | Common B-ALL | B-other (BCR::JAK2) | n. d. | De novo BCP-ALL | 79.3 | 35.5 | 49 | 74 |
| BCP-ALL 15 | 7 | F | 115 | No | No | Pre–B-ALL | B-other (P2RY8::CRLF2) | JAK2 (p.R683G) | De novo BCP-ALL | 99.5 | 81.5 | 76 | 200∗∗ |
| T-ALL 1 | 2 | M | 672 | Yes | Yes | Pre–T-ALL | — | STAT5B (p.N642H) | De novo T-ALL | 62.6 | 78.62 | 38 | 71 |
| T-ALL 2 | 7 | M | 872 | No | No | Cortical T-ALL | — | NOTCH1 (p.V1676D) / NRAS (p.Y64N) | De novo T-ALL | 59.80 | 88.83 | 136 | 211 |
| T-ALL 6 | 4 | M | 15.8 | Yes | Yes | Mature T-ALL | — | n. d. | R/R T-ALL | 62.90 | 39.86 | 57 | 80 |
| T-ALL 9 | 27 | F | 3.4 | Yes | Yes | ETP-ALL | — | NRAS (p.G12C) PTEN (p.R142Q) NOTCH1 (p.L1574P) | De novo T-ALL (adult) | 32.10 | 32.60 | 122 | 147 |
| T-ALL 10 | 4 | M | 10.23 | Yes | Yes | Pre–T-ALL | — | n. d. | R/R T-ALL | 36.90 | 20.52 | 50 | 71 |
| T-ALL 11 | 8 | F | 170.9 | Yes | Yes | Cortical T-ALL | — | NOTCH1 (p.L1600P) | R/R T-ALL | 77.3 | 35.22 | 37 | 57 |
| T-ALL 12 | 2 | F | 550 | Yes | No | Cortical T-ALL | — | n. d. | R/R T-ALL | 20.40 | 24.20 | 38 | 80 |
| T-ALL 13 | 11 | M | 226.5 | Yes | Yes | Mature T-ALL | — | n. d. | R/R T-ALL | 63.20 | 48.10 | 39 | 49 |
| T-ALL 14 | 9 | M | 36.3 | Yes | No | Cortical T-ALL | — | n. d. | R/R T-ALL | 26.60 | 18.05 | 38 | 60 |
| Patient characteristics . | PDX data . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDX no. . | Age at diagnosis, y . | Sex . | White blood cell count/µl . | Relapse . | Death . | Immunophenotype . | Cytogenetics∗ . | Relevant mutations† . | Sample origin . | CD127+ cells,‡ % . | Delta PBB§ . | Survival control‖ . | Survival LUSV¶ . |
| BCP-ALL 1 | 10.4 | M | 7.8 | No | No | Common B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 65 | 68.42 | 84 | 129 |
| BCP-ALL 2 | 14.8 | M | 26.8 | No | No | Pre-B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 83.4 | 63.25 | 99 | 227 |
| BCP-ALL 3 | 8.6 | F | 12.6 | No | No | Common B-ALL | TCF3::PBX1 | NRAS (p.G13D) | De novo BCP-ALL | 45.9 | 25.9 | 59 | 60 |
| BCP-ALL 4∗∗ | 13.4 | M | 12.32 | Yes | Yes | Pre-B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 67.7 | 52.79 | 56 | 85 |
| BCP-ALL 5∗∗ | 13.4 | M | 12.32 | Yes | Yes | Pre–B-ALL | TCF3::PBX1 | n. d. | R/R BCP-ALL | 77.7 | 74.4 | 65 | 111 |
| BCP-ALL 6 | 17.3 | F | 13.3 | Yes | No | Common B-ALL | BCR::ABL1 | n. d. | De novo BCP-ALL | 65.5 | 68.4 | 58 | 135 |
| BCP-ALL 7 | 9.8 | F | 132 | No | No | Common B-ALL | BCR::ABL1 | n. d. | De novo BCP-ALL | 38.2 | 46 | 61 | 62 |
| BCP-ALL 8 | 9 | M | 3.5 | No | No | Common B-ALL | BCR::ABL1 | n. d. | De novo BCP-ALL | 59.4 | 51.6 | 84 | 119 |
| BCP-ALL 9 | 0.7 | F | 103 | No | No | Pre–B-ALL | KMT2A::MLLT1 | n. d. | De novo BCP-ALL | 45.1 | 24.9 | 55 | 62 |
| BCP-ALL 10 | 1.2 | M | 60.8 | Yes | Yes | Common B-ALL | KMT2A::MLLT10 | n. d. | De novo BCP-ALL | 29.8 | 30.9 | 37 | 44 |
| BCP-ALL 11 | 1.6 | M | 288.4 | No | No | B-ALL | B-other# (EBF1::PDGFRB) | n. d. | De novo BCP-ALL | 37.5 | 2 | 88 | 97 |
| BCP-ALL 12 | 3.1 | F | 103.53 | Yes | No | Common B-ALL | B-other (DNMT3B::PAX5) | IL7R (p.L243_T244delinsRCGI) (GoF) | De novo BCP-ALL | 56.6 | 73.18 | 83 | 228 |
| BCP-ALL 13 | 10.7 | M | 113 | No | No | Common B-ALL | TCF3::PBX1 | n. d. | De novo BCP-ALL | 86.8 | 56.11 | 77 | 100 |
| BCP-ALL 14 | 5 | M | 204 | Yes | Yes | Common B-ALL | B-other (BCR::JAK2) | n. d. | De novo BCP-ALL | 79.3 | 35.5 | 49 | 74 |
| BCP-ALL 15 | 7 | F | 115 | No | No | Pre–B-ALL | B-other (P2RY8::CRLF2) | JAK2 (p.R683G) | De novo BCP-ALL | 99.5 | 81.5 | 76 | 200∗∗ |
| T-ALL 1 | 2 | M | 672 | Yes | Yes | Pre–T-ALL | — | STAT5B (p.N642H) | De novo T-ALL | 62.6 | 78.62 | 38 | 71 |
| T-ALL 2 | 7 | M | 872 | No | No | Cortical T-ALL | — | NOTCH1 (p.V1676D) / NRAS (p.Y64N) | De novo T-ALL | 59.80 | 88.83 | 136 | 211 |
| T-ALL 6 | 4 | M | 15.8 | Yes | Yes | Mature T-ALL | — | n. d. | R/R T-ALL | 62.90 | 39.86 | 57 | 80 |
| T-ALL 9 | 27 | F | 3.4 | Yes | Yes | ETP-ALL | — | NRAS (p.G12C) PTEN (p.R142Q) NOTCH1 (p.L1574P) | De novo T-ALL (adult) | 32.10 | 32.60 | 122 | 147 |
| T-ALL 10 | 4 | M | 10.23 | Yes | Yes | Pre–T-ALL | — | n. d. | R/R T-ALL | 36.90 | 20.52 | 50 | 71 |
| T-ALL 11 | 8 | F | 170.9 | Yes | Yes | Cortical T-ALL | — | NOTCH1 (p.L1600P) | R/R T-ALL | 77.3 | 35.22 | 37 | 57 |
| T-ALL 12 | 2 | F | 550 | Yes | No | Cortical T-ALL | — | n. d. | R/R T-ALL | 20.40 | 24.20 | 38 | 80 |
| T-ALL 13 | 11 | M | 226.5 | Yes | Yes | Mature T-ALL | — | n. d. | R/R T-ALL | 63.20 | 48.10 | 39 | 49 |
| T-ALL 14 | 9 | M | 36.3 | Yes | No | Cortical T-ALL | — | n. d. | R/R T-ALL | 26.60 | 18.05 | 38 | 60 |
ETP, Early T-cell precursor; F, female; M, male; n. d., none detected.
Stratification relevant lesions were diagnosed by fluorescence in situ hybridization or targeted RNA sequencing as described previously.31
Mutations were detected via targeted DNA sequencing using a custom DNA hybridization panel covering 151 genes frequently mutated in hematologic malignancies as described previously.31
CD127+ cells of all cells in the ALL cell population.
Reduction of peripheral blood blasts (PBB) of LUSV-treated mice compared with control-treated mice by the time one of the corresponding PDX animals or animal groups showed signs of overt leukemia (ΔPBB = PBBControl-treated – PBBLUSV-treated).
Survival of control–treated PDX mouse.
Survival of LUSV–treated PDX mouse.
B-other: BCP-ALL cases without the stratification relevant translocations ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, KMT2A-rearrangements.
Matched pair obtained from initial diagnosis and relapse.
LUSV is effective in overt leukemia PDX models. (A-C) A phase 2-like PDX study was performed using 24 PDX samples of patients with BCP-ALL and T-ALL with different CD127 expression levels including 8 samples from relapsed/refractory (R/R) disease. Two NSG mice per patient were injected with PDX cells, randomly assigned into treatment groups, and LUSV therapy was initiated upon detection of 1% PDX cells in the PB, modeling an overt leukemia situation. (A) Blood of both control and LUSV-treated animals bearing the same PDX sample was withdrawn when 1 of the 2 PDX mice showed signs of overt leukemia, and the number of hCD45+/hCD19+/mCD45– cells in the PB was measured via flow cytometry. The waterfall plot shows the difference in PBBs between respective control and LUSV-treated mice (ΔPBB, sorted from weakest therapy response to highest therapy response). Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. The dotted line indicates a ΔPBB of 50%. The white asterisk indicates matched samples obtained from initial diagnosis and relapse from the same patient. (B) Therapy-associated differences in the survival of NSG mice were determined using Kaplan-Meier log-rank statistics. The experiment was terminated after 280 days, and 1 LUSV–treated PDX animal was found free of leukemia. (C) The in vivo response to LUSV therapy as depicted via the ΔPBB value was correlated with the ratio of CD127+ cells in corresponding PDX samples determined via flow cytometry, Pearson linear regression, confidence interval (0.4010-0.8565). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
LUSV is effective in overt leukemia PDX models. (A-C) A phase 2-like PDX study was performed using 24 PDX samples of patients with BCP-ALL and T-ALL with different CD127 expression levels including 8 samples from relapsed/refractory (R/R) disease. Two NSG mice per patient were injected with PDX cells, randomly assigned into treatment groups, and LUSV therapy was initiated upon detection of 1% PDX cells in the PB, modeling an overt leukemia situation. (A) Blood of both control and LUSV-treated animals bearing the same PDX sample was withdrawn when 1 of the 2 PDX mice showed signs of overt leukemia, and the number of hCD45+/hCD19+/mCD45– cells in the PB was measured via flow cytometry. The waterfall plot shows the difference in PBBs between respective control and LUSV-treated mice (ΔPBB, sorted from weakest therapy response to highest therapy response). Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. The dotted line indicates a ΔPBB of 50%. The white asterisk indicates matched samples obtained from initial diagnosis and relapse from the same patient. (B) Therapy-associated differences in the survival of NSG mice were determined using Kaplan-Meier log-rank statistics. The experiment was terminated after 280 days, and 1 LUSV–treated PDX animal was found free of leukemia. (C) The in vivo response to LUSV therapy as depicted via the ΔPBB value was correlated with the ratio of CD127+ cells in corresponding PDX samples determined via flow cytometry, Pearson linear regression, confidence interval (0.4010-0.8565). ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
These data indicate that LUSV may be effective in overt BCP- and T-ALL, including HR and R/R leukemias. The level of surface CD127 expression may represent a predictive biomarker for LUSV response in patients with ALL.
LUSV efficacy is enhanced in combination with standard polychemotherapy
Antibody-based immunotherapy is commonly administered in combination with chemotherapy. Hence, we next tested the effect of combining LUSV treatment with standard-of-care polychemotherapy. A phase 2-like PDX overt leukemia study was conducted testing LUSV alone or in combination with dexamethasone/vincristine/PEG-asparaginase, mimicking ALL induction therapy.23,25,27 Nine CD127hi PDX samples (6 × BCP-ALL and 3 × T-ALL PDX; Figure 4A; supplemental Table 2) were used. As expected, LUSV alone prolonged the survival of PDX mice in comparison with control (median, 106 days vs 79 days; P = .0071). The effect of LUSV monotherapy was comparable with the effect of polychemotherapy (median, 106 days vs 79 days; P = .0187; Figure 4B). The strongest effect was observed when combining LUSV with polychemotherapy (median, 79 days for control vs not reached for combination; P < .0001; Figure 4B). Overall, 5 of 9 (56%) PDX mice treated with the combination survived the experiment and were MRD negative upon termination (Figure 4C). These data support the hypothesis that polychemotherapy regimen may enhance the efficacy of IL-7R immunotherapy, thus further promoting the clinical relevance and applicability of LUSV treatment in ALL.
LUSV enhanced activity in combination with polychemotherapy. (A-C) A phase 2-like PDX study was performed using 9 PDX samples of patients with BCP-ALL and T-ALL with high CD127-expression levels (CD127hi). Mice were left untreated (control) or treated with LUSV, ALL induction-like chemotherapy (Chemo), or the combination (Combi). Chemotherapy comprised vincristine, dexamethasone, and PEG-asparaginase cycles as previously published.23,25,27 (A) Schematic depiction of the study setup. (B) Survival was analyzed using the Kaplan-Meier method and log-rank statistics. The P value was adjusted using the Bonferroni method, considering k = 6 comparisons. Hence, a P value of () = .0083 was considered at statistically significant. (C) The experiment was terminated after 260 days, and BM samples of sacrificed animals were analyzed for MRD by PCR for patient–specific Ig/T-cell receptor rearrangements. Overt ALL, mouse showed clinical signs of leukemia upon euthanasia. neg, negative (below detection limit); ns, not significant; n.r. = median survival not reached; PCR, polymerase chain reaction; Pos, MRD positivity (MRD level ≥10–3); neg, MRD negativity (MRD level <10–3).
LUSV enhanced activity in combination with polychemotherapy. (A-C) A phase 2-like PDX study was performed using 9 PDX samples of patients with BCP-ALL and T-ALL with high CD127-expression levels (CD127hi). Mice were left untreated (control) or treated with LUSV, ALL induction-like chemotherapy (Chemo), or the combination (Combi). Chemotherapy comprised vincristine, dexamethasone, and PEG-asparaginase cycles as previously published.23,25,27 (A) Schematic depiction of the study setup. (B) Survival was analyzed using the Kaplan-Meier method and log-rank statistics. The P value was adjusted using the Bonferroni method, considering k = 6 comparisons. Hence, a P value of () = .0083 was considered at statistically significant. (C) The experiment was terminated after 260 days, and BM samples of sacrificed animals were analyzed for MRD by PCR for patient–specific Ig/T-cell receptor rearrangements. Overt ALL, mouse showed clinical signs of leukemia upon euthanasia. neg, negative (below detection limit); ns, not significant; n.r. = median survival not reached; PCR, polymerase chain reaction; Pos, MRD positivity (MRD level ≥10–3); neg, MRD negativity (MRD level <10–3).
LUSV promotes antileukemic efficacy by direct antagonist effects on IL-7R signaling and induction of ADCP
Next, we characterized the antileukemic mode of action of LUSV. First, we tested the ability of LUSV to block IL-7-induced in vitro survival and proliferation and found that LUSV prevented the IL-7 prosurvival and proproliferative effect on CD127+ PDX cells (Figure 5A-B). IL-7–induced expansion of sorted healthy CD3+ T lymphocytes (98% CD127+ cells) was mildly affected by LUSV (13.6-fold vs 10.9-fold T-cell increase; supplemental Figure 4A-B). Sorted CD19+ B lymphocytes weakly expressed CD127 (5% CD127+ cells) and did not expand in response to IL-7 stimulation (supplemental Figure 4C-D). Secondly, we investigated the effect of LUSV on IL-7–induced signaling by monitoring P-STAT5 levels in leukemic cells. In ALL cell lines and PDX specimens that responded to IL-7 stimulation with increased P-STAT5 levels (>10% P-STAT5+ cells, referred to as “IL-7 responsive”; supplemental Table 2), LUSV efficiently reverted this activation and reduced P-STAT5 levels in a dose-dependent fashion (Figure 5C).
LUSV exhibits direct antileukemic efficacy in ALL cells. Treatment effects of LUSV and IL-7 on leukemic cell survival (A) and proliferation of 2 representative T-ALL PDX specimens (36.2% and 84.1% CD127+ cells, respectively) (B). (C) Effect of LUSV treatment on the level of IL-7 induced STAT5 phosphorylation (P-STAT5) in leukemic cells, identifying IL-7 responsive and unresponsive T- and BCP-ALL cell lines and PDX cells, as indicated (also compare supplemental Table 2). (D) Immunodeficient mice were transplanted with PDX cells from an TCF3::PBX1+ patient (83.4% CD127+ blasts) and treated with LUSV (5 mg/kg) or a control vehicle (n = 10, respectively) starting when 1% PDX cells were detected in the PB (IV treatment on day +1, +3, +7, +14 and every 14 days thereafter). Survival was analyzed using the Kaplan-Meier method and log-rank statistics.
LUSV exhibits direct antileukemic efficacy in ALL cells. Treatment effects of LUSV and IL-7 on leukemic cell survival (A) and proliferation of 2 representative T-ALL PDX specimens (36.2% and 84.1% CD127+ cells, respectively) (B). (C) Effect of LUSV treatment on the level of IL-7 induced STAT5 phosphorylation (P-STAT5) in leukemic cells, identifying IL-7 responsive and unresponsive T- and BCP-ALL cell lines and PDX cells, as indicated (also compare supplemental Table 2). (D) Immunodeficient mice were transplanted with PDX cells from an TCF3::PBX1+ patient (83.4% CD127+ blasts) and treated with LUSV (5 mg/kg) or a control vehicle (n = 10, respectively) starting when 1% PDX cells were detected in the PB (IV treatment on day +1, +3, +7, +14 and every 14 days thereafter). Survival was analyzed using the Kaplan-Meier method and log-rank statistics.
Yet, we identified several ALL cases as “IL-7 unresponsive”: The T-ALL cell line DND41 showed constitutively active P-STAT5 (due to an IL-7R GoF mutation), whereas in some ALL samples, IL-7 stimulation did not induce P-STAT5 (<10% P-STAT5+ cells; supplemental Table 2) despite high CD127 expression (Figure 5C). To investigate whether LUSV can also be effective in such specimens, we treated mice bearing an IL-7 unresponsive PDX sample of an TCF3::PBX1+ patient with LUSV in an overt leukemia setting (BCP-ALL PDX number 2, 83.4% CD127+ cells; supplemental Figure 4B; Table 1). Indeed, we observed strong antileukemic in vivo activity and a survival prolongation in response to LUSV (median, 99 vs 227 days; P < .0001; Figure 5D). This observation suggests that LUSV is also effective in IL-7–unresponsive ALL and induces antileukemic activity by mechanisms other than IL-7R pathway blockade.
Therapeutic antibodies may also induce immunological effector functions such as ADCC or ADCP.31 LUSV is an IgG4 antibody.15 IgG4 isotypes have limited effector functions and predominantly act via recruitment of Fc-gamma receptor 1+ (CD64+) effector cells such as macrophages.32 Accordingly, LUSV did not promote ADCC in healthy T lymphocytes (supplemental Figure 5A-B). To test the ability of LUSV to induce ADCP, IL-7–responsive and –unresponsive ALL cell lines were subjected to ADCP assays in vitro using primary human macrophages as effector cells (Figure 6A). We observed that LUSV treatment induced macrophage-mediated ADCP in all CD127+ ALL cell lines in a dose-dependent manner (Figure 6B) but only marginally in healthy T lymphocytes and not at all in CD127– Jurkat cells and healthy sorted CD19+ B lymphocytes (Figure 6B; supplemental Figure 5C). Furthermore, LUSV-induced ADCP was low or absent in healthy T and B lymphocytes when combined with low dose chemotherapy (supplemental Figure 6A-F).
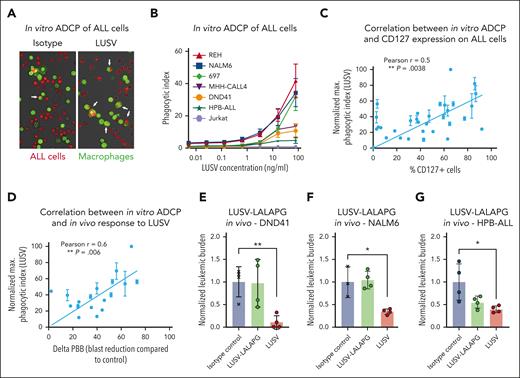
LUSV eradicates ALL cells via ADCP. (A) Representative images of an in vitro ADCP assay with primary human macrophages (labeled in green) on T-ALL PDX cells (labeled in red) after isotype control or LUSV treatment. (B) Level of in vitro human primary macrophage-mediated phagocytosis induced by increasing concentrations of LUSV on BCP- and T-ALL cell lines as indicated. Correlation between the level of in vitro THP1–mediated phagocytosis induced by LUSV treatment and (C) the level of CD127 expression on leukemic cells or with (D) in vivo efficacy of LUSV treatment (difference in PBBs between respective control and LUSV-treated mice, ΔPBB). Correlation P value and r were measured through the Pearson method. (E-G) Normalized in vivo leukemic burden after control, LUSV-LALAPG, or LUSV treatments in mice injected with either the T-ALL cell line DND41 (IL-7R GoF mutated) (E), the BCP-ALL cell line NALM6 (IL-7 unresponsive) (F), or the T-ALL cell line HPB-ALL (IL-7 responsive) (G). Differences in leukemic burden were assessed using the unpaired Student t test. ∗P < .05; ∗∗P < .01.
LUSV eradicates ALL cells via ADCP. (A) Representative images of an in vitro ADCP assay with primary human macrophages (labeled in green) on T-ALL PDX cells (labeled in red) after isotype control or LUSV treatment. (B) Level of in vitro human primary macrophage-mediated phagocytosis induced by increasing concentrations of LUSV on BCP- and T-ALL cell lines as indicated. Correlation between the level of in vitro THP1–mediated phagocytosis induced by LUSV treatment and (C) the level of CD127 expression on leukemic cells or with (D) in vivo efficacy of LUSV treatment (difference in PBBs between respective control and LUSV-treated mice, ΔPBB). Correlation P value and r were measured through the Pearson method. (E-G) Normalized in vivo leukemic burden after control, LUSV-LALAPG, or LUSV treatments in mice injected with either the T-ALL cell line DND41 (IL-7R GoF mutated) (E), the BCP-ALL cell line NALM6 (IL-7 unresponsive) (F), or the T-ALL cell line HPB-ALL (IL-7 responsive) (G). Differences in leukemic burden were assessed using the unpaired Student t test. ∗P < .05; ∗∗P < .01.
To further substantiate ADCP as a mechanism of action of LUSV, we measured the level of ADCP induction upon LUSV treatment in a variety of PDX cells (n = 24) using the phagocytic THP1 cell line as effector cells, which provide ADCP capacity correlating with that of primary macrophages (supplemental Figure 7A). A normalized phagocytic score was calculated, as previously reported.28,29 We observed a correlation between ADCP induced by LUSV and CD127 expression on leukemic cells (Pearson r = 0.5; P = .0066; Figure 6C). Accordingly, small hairpin RNA (shRNA)-mediated depletion of CD12712 prevented LUSV–mediated ADCP in 697 cells (supplemental Figure 7B). Importantly, the extent of in vitro ADCP correlated with the in vivo response to LUSV in our overt leukemia phase 2-like PDX study (Pearson r = 0.6; P = .006; Figure 6D).
We next generated a LUSV variant harboring Fc-inactivating mutations,33 further referred to as “LUSV-LALAPG” (supplemental Figure 7C). LUSV-LALAPG bound to CD127 and inhibited IL-7–induced P-STAT5 similarly to LUSV (supplemental Figure 7D-E) but, as expected, lacked the capacity to induce primary macrophage-mediated ADCP on CD127+ leukemic cells (supplemental Figure 7F).
We then assessed the in vivo efficacy of LUSV-LALAPG on the IL-7–unresponsive cell lines DND41 and NALM6. In both cases, LUSV-LALAPG failed to reduce the leukemic burden of xenografted mice, as opposed to LUSV, which induced a significant reduction of leukemic burden (Figure 6E-F). Finally, we investigated the in vivo efficacy of LUSV-LALAPG on the IL-7–responsive cell line HPB-ALL (supplemental Figure 7D). LUSV-LALAPG reduced the leukemic burden of mice xenografted with this cell line, however to a lesser extent than LUSV (Figure 6G). These observations illustrate that LUSV acts via IL-7R pathway blockade in IL-7–responsive ALL but also induces ADCP alone in IL-7–unresponsive ALL. Mouse body weight was not affected upon LUSV therapy in any models (supplemental Figure 7G-I).
Overall, our data demonstrate high antileukemic efficacy of LUSV treatment in vivo through a dual mode of action relying on IL-7R signaling blockade in IL-7–responsive leukemias and induction of ADCP in CD127-expressing leukemias, irrespective of IL-7R pathway activity, and may therefore be considered a therapeutic option for a large fraction of patients with ALL.
Discussion
Relapsed disease and toxicity of polychemotherapy remain major challenges in the treatment of ALL. New immunotherapy options are urgently needed for targeted ALL therapy, especially for R/R ALL.34 Numerous recent studies show the IL-7R pathway to be critical for ALL development5,6,10,35,36 and its exploitability for ALL treatment.12-14,37,38 Preclinical data suggest that direct targeting of IL-7R in ALL cells using research-grade CD12 mAbs outperforms inhibitors of IL-7R downstream effectors such as ruxolitinib (targeting JAK/STAT signaling).12
In this respect, different mAb constructs directly targeting IL-7R have been proposed and evaluated for preclinical efficacy in BCP- and T-ALL.13,14 Yet, none of these potential therapeutics has advanced to systematic clinical testing. Here, we show that the clinical grade CD127 mAb LUSV efficiently targets BCP- and T-ALL cells in PDX models as monotherapy and especially when combined with chemotherapy via a dual mode of action comprising IL-7R pathway blockade and ADCP.
Despite murine studies indicating that IL-7R targeting leads to lymphopenia, recent phase 1 trials show that CD127-targeting mAbs, including LUSV are a safe therapeutic approach.18,39 LUSV did not induce T-cell compartment alterations in cynomolgus monkeys even at escalating doses up to 100 mg/kg.15 Moreover, IV dosing of 10 mg/kg twice did not lead to adverse events, significant lymphopenia, or lymphocyte compartment alterations in healthy individuals.18 This is in line with our observation that LUSV–induced lower levels of ADCP in healthy lymphocytes than leukemia cells. It can be hypothesized that although expressing substantial levels of surface CD127, healthy lymphocytes do not expose an altered composition of “eat-me” (eg, calreticulin) and “don’t eat-me” (eg, CD47 and CD24) signals as their malignant counterparts. Yet, further studies must be conducted to substantiate this. Furthermore, the absence of toxicity toward healthy lymphocytes in vivo remains to be thoroughly investigated in ALL, to elucidate whether LUSV may be used in future phase 1/2 clinical trials in patients with ALL.
Recently proposed IL-7R antagonist mAbs with reported preclinical efficacy in ALL include a humanized IgG1 antibody14 and a fully human IgG1 antibody,13 which both rely on IL-7R blockade and ADCC. Due to internalizing features, the latter also works as antibody-drug conjugate. By contrast, LUSV is not internalized15 and therefore represents a stable interface for effector cell recruitment.
Because LUSV is an IgG4 isotype, its mode of action fundamentally differs from other proposed IL-7R targeting mAbs. IgG4-mAbs like LUSV predominantly act via CD64 binding on effector cells thus representing less potent agents than IgG1-mAbs, which promote ADCC and complement-dependent cytotoxicity.15,40 On the contrary, this limited capacity to induce effector functions may underly the excellent safety profile of LUSV.18
For tailored mAb therapy, it is crucial to anticipate patients who may benefit from a particular antibody. The observation that the in vitro and in vivo efficacies of LUSV were in clear correlation with CD127 surface expression and that ∼40% of patients with ALL exposed high CD127 expression indicates that a large fraction of patients with BCP-ALL and T-ALL could be eligible for IL-7R immunotherapy. Accordingly, strongest and in some cases even curative in vivo effects by LUSV alone were observed in BCP-ALL and T-ALL PDX samples harboring mutations that fuel IL-7R signaling such as NOTCH19 (T-ALL PDX number 2 and 8), STAT58 (T-ALL PDX number 1), JAKs36,41 (BCP-ALL PDX number 15), as well as IL-7R itself (BCP-ALL PDX number 12).36,42 Hence, CD127 surface expression can be considered a predictive biomarker for the efficacy of LUSV and potentially other IL-7R targeting antibodies in patients with ALL, which is easily and reliably assessable via flow cytometry. IL-7R threshold levels for clinical efficiency of IL-7R immunotherapy are yet to be defined. Our results suggest that CD127high ALL cases should be prioritized for LUSV therapy, omitting rare cases with bimodal CD127 expression.
Our data indicate that ALL subgroups with high clinical need could benefit from IL-7R immunotherapy: we detected high CD127 levels in T-ALL cases and all tested T-ALL PDX samples exposed in vivo response to LUSV, including samples obtained from R/R and adult patients. Moreover, TCF3::PBX1+ BCP-ALL cases exposed particularly high CD127 expression. This is in line with previous work suggesting an association between high IL-7R levels and poor prognosis in this subgroup.12,43 Accordingly, TCF3::PBX1+ PDX samples responded well to IL-7R therapy including 1 matched sample of initial diagnosis and relapsed disease (BCP-ALL PDX 4-5), which is important because TCF3::PBX1+ BCP-ALL relapse is, to date, virtually incurable.44 In line with previous reports, we observed high IL-7R expression in IKZF+ ALL cases, and IKZF1 represents a key suppressor of IL-7R signaling.45 The IKZF+ signature defines MRD-HR BCP-ALL and is associated with BCR::ABL1+ and BCR::ABL1–like BCP-ALL.46,47 In the latter subgroup, which has an adverse prognosis, we detected high IL-7R levels in CRLF2-fusion–positive ALL and marked response to LUSV in a P2RY8::CRLF2-fusion–positive PDX specimen in vivo.48 Additionally, a recent report showed comparable expression levels of CD127 in pediatric and adult patients, suggesting that both groups may benefit from IL-7R immunotherapy.38
Due to its dual mode of action, particular efficacy of LUSV may be anticipated for those ALL cases that simultaneously expose both high IL-7R expression and IL-7R pathway dependency. This is strengthened by our in vivo data that show strongest LUSV efficacy when ADCP and direct effects are triggered.
Importantly, a functional (myeloid) effector cell compartment will be needed for LUSV to fulfill its ADCP–driven antileukemic activity, which may be disturbed in patients with pretreated ALL.49
We and others observed that the efficacy of IL-7R immunotherapy can be augmented when combined with chemotherapy.13,14 This holds true even in CD127low PDX mice and when administered sequentially (postchemotherapy MRD). This may be to some extent due to enhanced glucocorticoid efficacy upon IL-7R pathway blockade.50 Chemotherapy may induce the expression of certain “eat-me” signals on tumor cells that together with ADCP-promoting features of LUSV may potentiate the phagocytosis of ALL cells.51,52 We observed no significant ADCP induction when combining LUSV with low dose chemotherapy in physiological B and T cells and no additional signs of toxicity in our in vivo models.
One way to further enhance the efficacy of IL-7R immunotherapy could be combination therapies simultaneously targeting IL-7R and other related pathways. In that respect, we previously showed that IL-7R expression strongly correlated with the expression of pre–B-cell receptor molecules CD79a/b in BCP-ALL, which can also efficiently be targeted with therapeutic antibodies.21,22 Moreover, we showed profound preclinical efficacy of CD47 antibodies in T-ALL and BCP-ALL.23,53 Because both LUSV and CD47-blocking antibodies exploit ADCP as major mechanism of action, a particular synergy may be expected when combining these compounds.
Altogether, we show preclinical efficacy of a nonlymphodepleting and ADCP-promoting CD127 mAb in heterogeneous models of BCP-ALL and T-ALL. Our data suggest LUSV as a novel and safe immunotherapeutic agent for the treatment of BCP- and T-ALL, particularly in R/R disease and in combination with chemotherapy, warranting clinical investigation.
Acknowledgments
The authors thank the patients and physicians who contributed samples and data for this study. The authors thank Gabriele Riesen, Birthe Fedders, Martina Kähler, Evelin Kerner, Ann-Kathrin Holztmann,Katrin Neumann, Katrin Timm-Richert, and Sabine Prigge for the excellent technical assistance. The authors thank Silvia Comis, Véronique Blanc, Dennis Das Gupta, and Johanna Horns for insightful scientific discussions and support throughout the study.
L.L. and J.H. have been supported by the Faculty of Medicine, University of Kiel, Kiel, Germany. L.L., D.M.S., M.P., G.C., M.S., and M.B. are members of the Clinical Research Unit “CATCH ALL” (KFO 5010/1) funded by the Deutsche Forschungsgemeinschaft, Bonn, Germany.
Authorship
Contribution: L.L. and I.B. designed and performed experiments, analyzed data and wrote the manuscript; J.H., C.R., A.D., D.W., C.M., E.N., J.T., S.N., M.D., S.P., F.V., V.D., T.S., and W.W. performed experiments and analyzed data; M.S., G.C., B.B., J.-P.B., S.R., G.E., M.L.d.B., and J.M.B. provided ALL samples and clinical data; Ž.A. and A.K.B. provided genomic and transcriptomic data; J.A. guided and assessed MRD analysis; M.P. provided ADCP analysis and scientific counseling; A.L. and M.B. conceived and performed prospective flow cytometry analysis; D.M.S., F.C., N.P., L.L., and I.B. initiated and designed the study and discussed the research direction; and all authors discussed the manuscript.
Conflict-of-interest disclosure: L.L. received research funding from OSE Pharmaceuticals. M.B. received consulting fees from PRMA; research funding from Amgen; honoraria from Novartis, Pfizer, and Amgen; and was an advisory board member for Incyte and Amgen. D.M.S. was an advisory board member for Bayer, SOBI, and Jazz Pharmaceuticals; and received research funding from OSE Pharmaceuticals. M.S. received research funding from Shire and Servier; and fees for advisory board functions from Jazz Pharmaceuticals and Servier. F.C., I.B., E.N., J.T., S.P., and N.P. are employees and F.C., I.B., and N.P are shareholders of OSE Immunotherapeutics, a company owning the OSE-127 anti-IL-7Ra antagonist mAb; they are authors of patents related to anti-IL-7Ra antagonist mAb. The remaining authors declare no competing financial interests.
Correspondence: Lennart Lenk, Department of Pediatrics I, ALL-BFM Study Group, Christian-Albrecht University Kiel and University Medical Center Schleswig-Holstein, Rosalind-Franklin-Str 9, Quincke-Forschungszentrum, 24105 Kiel, Germany; email: lennart.lenk@uksh.de; Denis M. Schewe, Otto von Guericke-University Magdeburg, Medical Faculty, Leipziger Str, House 65, 39120 Magdeburg, Germany; email: denis.schewe@med.ovgu.de; and Irène Baccelli, OSE Immunotherapeutics, 22 Boulevard Benoni Goullin, 44200 Nantes, France; email: baccelli@gmail.com.
References
Author notes
L.L. and I.B. contributed equally to this study.
N.P. and D.M.S. are joint senior authors.
Parts of this study have been presented at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11-14 December 2021, the 64th annual meeting of the American Society of Hematology, New Orleans, LA, 10-13 December 2022, and the 2023 annual meeting of the American Association for Cancer Research, Orlando, FL, 14-19 April 2023.
All data supporting the findings of this study are available within the article and its supplementary information files.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![CD127 is expressed in the majority of BCP-ALL and T-ALL cases. CD127 surface expression was prospectively measured via flow cytometry in 371 diagnostic blood or BM samples of patients with BCP-ALL and T-ALL in accordance with International-BFM-FLOW recommendations.19,20 CD127 low positivity was defined as ≥10% CD127+ ALL cells by flow cytometry and high expression as ≥50% CD127+ blasts within the CD45dim/CD19+ (BCP-ALL) or CD45dim/CD7+ (T-ALL) cell population, respectively. (A-C) Pie charts depicting the ratio of patients with CD127-negative, CD127-low, and CD127-high ALL among all analyzed patient samples (A), BCP-ALL (B), and T-ALL cases only within the cohort (C). (D) Comparison of percentage of CD127+ ALL cells in BCP-ALL vs T-ALL cases; ∗∗P = .0023, unpaired 2-sided t test. (E) Ratio of CD127+ ALL cells in different BCP-ALL subgroups. The stratification relevant lesions ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, and KMT2A-rearrangements (KMT2A-r) were diagnosed by fluorescence in situ hybridization or RNA sequencing. Further genetic alterations (CRLF2-rearrangements [CRLF2-r], TP53-mutations [TP53-mut], and ZNF384-fusions [ZNF384]) were detected within the “B-others” subgroup (BCP-ALL cases without ETV6::RUNX1, BCR::ABL1, TCF3::PBX1, and KMT2A-r). Blue lines show the cutoffs for low and high CD127-positivity. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/26/10.1182_blood.2023021088/3/m_blood_bld-2023-021088-gr1.jpeg?Expires=1768803273&Signature=rP1Jif5RayXKUXhoVaZ654IM4S3dmJy7tB5lOIL5XPNNO7grw8M989hqMrqME0F0EWWES-Pq28MTSAfoq42YeHiYqDJPD5WvB1t0IuFR7AvyXv6e7YPQVyI8HENUu5UOWBN2wNrWglHoa9w3sOSDSIiSWKcHm7to9vA-nfeDkRHDkAs7fFtTtnkrIy5EWXtz6i-zAnxpMX-w46ds3dlfYR5veeQSIECfwPbjhvcd9YeNPQdM6ePu7ns2l0bSOsa8BNIe0UsbVffWehBwQw2v0YXR2ije4lNC0pXe-sZRDnQ3vFhKS~OoZ1RnJfDPK-LAGC1RTlSUMViyQsJ~6aWVwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![LUSV reduces leukemic burden in MRD in vivo models of BCP-ALL and T-ALL. (A-C) Immunodeficient mice were transplanted with PDX cells from 2 different TCF3::PBX1+ patients (63.0% and 89.6% CD127+ blasts, respectively) and treated with LUSV (5 mg/kg) or a control vehicle (n = 10, respectively) starting the day after injection, modeling an MRD-like situation (IV treatment on day +1, +3, +7, and +14 and every 14 days thereafter as described previously12). (A) PB of both, control and LUSV-treated animals was withdrawn when the first PDX mouse showed signs of overt leukemia and compared for the ratio of hCD45+/hCD19+/mCD45– cells in the PB as measured via flow cytometry, unpaired 2-sided t test. Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. (B) Therapy-associated differences in the survival of NSG mice were determined using Kaplan-Meier log-rank statistics. (C) The experiment was terminated after 170 days, and BM samples of euthanized animals were analyzed for MRD by PCR for patient–specific Ig/T-cell receptor rearrangements. Overt ALL = mouse showed clinical signs of leukemia upon euthanization; neg, negative (below detection limit). (D-F) A phase 2-like PDX study was performed using CD127-high (≥50% CD127+ cells) T-ALL PDX samples (n = 8 patients) including 3 samples from R/R disease. Two NSG mice per patient were injected with PDX cells, randomly assigned into treatment groups and LUSV therapy was conducted in an MRD-like setting. (D) Experimental setup and therapy scheme. (E) Blood of both, control and LUSV-treated animals bearing the same PDX sample was withdrawn when one of the 2 PDX mice showed signs of overt leukemia and the number of hCD45+/hCD19+/mCD45– cells in the PB was measured via flow cytometry. The waterfall plot shows the difference in PBBs between respective control and LUSV-treated mice (sorted from weakest therapy response to highest therapy response). Animals not showing clinical signs of overt leukemia or >70% PBBs at this time point received further treatment until reaching termination criteria. (F) Therapy-associated differences in the survival of NSG mice was determined using Kaplan-Meier log-rank statistics. (G-H) Cells from 1 T-ALL PDX (T-ALL PDX number 1) were injected into replicate mice (n = 3 for no-treatment group [control]; n = 5 for treatment groups), animals were subjected to chemotherapy upon 10% PBBs and LUSV immunotherapy (chemotherapy + LUSV) vs mock treatment (chemotherapy only) was initiated upon reoccurrence of PBB (termed “postchemo-MRD” model).23 (G) Schematic depiction of the experimental setup of the postchemotherapy MRD model. (H) Therapy-associated differences in the survival of NSG mice was determined using Kaplan-Meier log-rank statistics. One mouse in the chemotherapy only group died due to procedural complications. The experiment was terminated after 130 days. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. n.r., not reached.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/26/10.1182_blood.2023021088/3/m_blood_bld-2023-021088-gr2.jpeg?Expires=1768803273&Signature=tSq9VsiBEcIPSkefLopXzl1mUsUR~H2dyhR7Q5bXOIAhdkp3amcrCVNcG5htrjptmISNTgcULJAGwiAKT1X9XZQ3W4tPpL5bB6JfQNdYeDR48VID6TjnUlenT2D3QuWDfoho7tSOEDNxnyKV9HsosQAyH4m0mLsTG4y0GRnZ~xPycYDjHm~gwj7EXSp3Q8ql0RJ4a5n5DbgDoNp6k~x2BDzHMEkLMJhM3Q-wl3LLAMejCr13emnoO26qpzDDQSHfG5T52RL0l16jntaYYlLBhFaEQ-muj9AA1iMjGXx9hiX5AmsNmYIY1t6FRebFAjRtxNkJYkXjSYwwu7e6UUV9bQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal