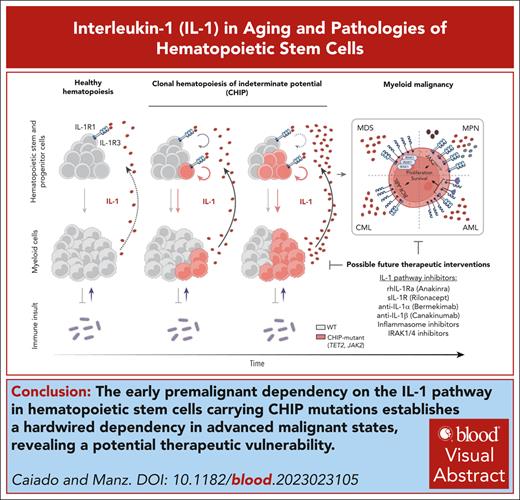
Visual Abstract
Defense-oriented inflammatory reactivity supports survival at younger age but might contribute to health impairments in modern, aging societies. The interleukin-1 (IL-1) cytokines are highly conserved and regulated, pleiotropic mediators of inflammation, essential to respond adequately to infection and tissue damage but also with potential host damaging effects when left unresolved. In this review, we discuss how continuous low-level IL-1 signaling contributes to aging-associated hematopoietic stem and progenitor cell (HSPC) functional impairments and how this inflammatory selective pressure acts as a driver of more profound hematological alterations, such as clonal hematopoiesis of indeterminate potential, and to overt HSPC diseases, like myeloproliferative and myelodysplastic neoplasia as well as acute myeloid leukemia. Based on this, we outline how IL-1 pathway inhibition might be used to prevent or treat inflammaging-associated HSPC pathologies.
Introduction
The hematopoietic system maintains lifelong blood cell production, essential for executing the primary functions of oxygen transportation (erythrocytes), hemostasis (platelets), and host defense (leukocytes). To maintain optimal activity and prevent life threatening conditions of insufficient or excessive hematopoietic cell output, the hematopoietic system is tightly regulated and organized in a hierarchical cellular differentiation system, sustained by a rare hematopoietic stem cell (HSC) population, which is kept in supportive bone marrow (BM) niches.1 Under steady-state conditions, most adult HSCs are quiescent and protected from the detrimental effects of proliferative stress.2,3 However, HSCs can sense environmental cues and initiate demand-adapted hematopoiesis by exiting quiescence and engaging in differentiating (generating multipotent progenitors [MPPs], lineage restricted progenitors, and mature cells) and self-renewing (generating progeny that retains HSC activity) cell divisions.4 The capacity to produce these 2 types of outcomes is an essential and exclusive feature of HSCs and allows the production of mature cells in demand, while maintaining the HSC pool size. HSC cellular fitness thus results from the interplay between these 2 properties and can be measured experimentally by assessing HSC repopulation capacity, for example, upon serial transplantation.
Inflammation is a protective immune response to infection and tissue damage, mediated by alarmins, cytokines, and chemokines produced by stressed cells and sensed by effector cells that orchestrate a systemic and/or local response aimed at restoring tissue homeostasis.5 A relevant aspect of the inflammatory response is the ability to reach the BM where it is sensed by hematopoietic stem and progenitor cells (HSPCs) and activates hematopoietic cell production. The interleukin-1 (IL-1) cytokines (IL-1α and IL-1β) are highly conserved and regulated mediators (alarmin and cytokine, respectively) of inflammatory responses, produced during defensive reactions with potent immune-amplifying effects and selected for in evolution for their advantageous role in control of infections and tissue damage. However, although defense-oriented inflammatory reactivity supports survival at younger age, it might contribute to aging-associated health alterations. In aging societies, enabled by more controlled environmental stressors and preventive and therapeutic medical interventions, long-term acting inflammatory consequences are becoming more evident.
Indeed, in recent years, new investigations have detailed how the IL-1 pathway interferes with HSC function and how this effect may contribute to HSC aging, dysfunction, and oncogenesis. Here, we summarize current knowledge in the field and highlight the potential of targeting the IL-1 signaling pathway in HSC pathologies.
IL-1 pathway
The IL-1 family is an evolutionary conserved group of proteins, composed of 11 soluble molecules and 10 receptors. The soluble molecules include pathway agonists (IL-1α/β, IL-18, IL-33, and IL-36a/b/g), antagonists (IL-1RN, IL-36RN, and IL-38) and an anti-inflammatory cytokine (IL-37). The IL-1 receptor (IL-1R) family members include agonistic (IL-1R1/3/4/5/6/7/9/10), antagonistic (IL-1R2/R8), and soluble receptors (sIL-1R1/2). Interestingly, different agonistic ligands use different receptors to promote pathway activation: IL-1α/β binds IL-1R1/R3; IL-18 binds IL-1R5/R7; IL-33 binds IL-1R4/R3; and IL-36 binds IL-1R6/R3 (extensively reviewed by Dinarello6 and Garlanda and Dinarello7). In this review we focus on IL-1α/β (collectively termed IL-1), which are the most extensively studied ligands of the IL-1 family and are potent proinflammatory cytokines. IL-1 ligation to IL-1R1 extracellular immunoglobulin-like domains triggers IL-1R1 association with its coreceptor IL-1R3 (also termed IL-1RAP) via their shared Toll–IL-1 resistance intracellular domain and recruit Toll–IL-1 resistance domain–containing adapter myeloid differentiation primary response protein 88 (MYD88). Further coupling to downstream kinases (eg, IL-1R–associated kinases [IRAKs] and tumor necrosis factor receptor–associated factor 6 [TRAF6]) leads to the activation of nuclear factor κB (NF-κB) signaling, p38, c-Jun N-terminal kinases (JNK), extracellular signal-regulated kinases (ERK), and mitogen-activated protein kinase (MAPK) pathways, which cooperatively, induce the expression of canonical IL-1 target genes (eg, IL-6, IL-8, MCP-1, COX-2, IκBα, MKP-1, IL-1α, and IL-1β) by transcriptional and posttranscriptional mechanisms.8 IL-1 pathway activation initiates a local inflammatory cascade that ultimately leads to recruitment/activation of both innate and adaptive immunity, mounting a defensive response against the initial immune insult9,10 (Figure 1, left panel). Importantly, although IL-1α and IL-1β trigger the same receptor and comparable downstream responses, they play essential and nonredundant roles in the context of in vivo bacterial infections. Indeed, IL-1β is necessary for pathogen clearance via granulocyte colony-stimulating factor–mediated granulopoiesis induction at infection sites and IL-1α is implicated in liver metabolic reprogramming in response to the infection. Accordingly, these different and complementary responses result from differential tissue expression levels of IL-1 in response to the infection, with IL-1α being more expressed by liver hepatocytes and IL-1β more expressed by splenic hematopoietic cells.11
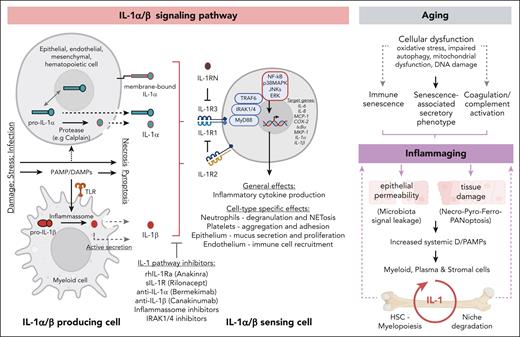
IL-1α/β signaling pathway as a driver of inflammaging. Left panel depicts the IL-1α/β signaling pathway: IL-1α is produced as a precursor protein (pro–IL-1α), which is constitutively expressed in multiple cell types (eg, keratinocytes, fibroblasts, and endothelial and epithelial cells) and shuttles between cytoplasm and nucleus, in which it acts as a transcription factor, functionally implicated in proliferation, senescence, and apoptosis. Upon cellular necrosis, cytoplasmatic pro–IL-1α is released together with cell contents acting as an “alarmin” in surrounding cells. Under inflammatory conditions, pro–IL-1α is cleaved into mature IL-1α that signals either as an extracellular or as a membrane bound molecule. IL-1β is primarily produced by myeloid cells in a precursor form. Upon danger/pathogen-associated molecular pattern (D/PAMP) recognition by pattern recognition receptors and downstream inflammasome-mediated proteolytic activation, pro-IL-1β matures into IL-1β. Secretion of IL-1β can occur via active secretion or via increased cellular permeability once cells go into pyroptosis. IL-1 engagement with IL-1R1/3 leads to downstream pathway activation, which can be prevented by naturally occurring inhibitors like soluble IL-1R1 antagonist (IL-1RN) and antagonistic receptor (IL-1R2, which lacks an intracellular signaling domain). IL-1 pathway inhibiting drugs are depicted. Recombinant human IL-1Ra (anakinra) targets both IL-1α and IL-1β, as does the soluble IL-1R (rilonacept). Neutralizing antibodies selectively target IL-1α (bermekimab) or IL-1β (canakinumab). General and cell-type specific effects of IL-1 signaling pathway activation are indicated. IL-1 pathway targeting compounds are indicated. Right panel depicts the interplay between aging, inflammaging and IL-1 signaling in the BM. Age associated molecular alterations result in the indicated cellular dysfunctions, which converge in inducing inflammaging. Inflammaging leads to multiple tissue alterations that result in D/PAMP production. D/PAMPs are sensed in the BM by myeloid, plasma, and stromal cells leading to increased IL-1 production, which, in turn, is sensed by niche cells and HSCs, triggering niche degradation and myelopoiesis, respectively. This cycle amplifies the local amounts of IL-1, leading in the long term to increased inflammatory burden, further amplifying the initial inflammaging levels.
IL-1α/β signaling pathway as a driver of inflammaging. Left panel depicts the IL-1α/β signaling pathway: IL-1α is produced as a precursor protein (pro–IL-1α), which is constitutively expressed in multiple cell types (eg, keratinocytes, fibroblasts, and endothelial and epithelial cells) and shuttles between cytoplasm and nucleus, in which it acts as a transcription factor, functionally implicated in proliferation, senescence, and apoptosis. Upon cellular necrosis, cytoplasmatic pro–IL-1α is released together with cell contents acting as an “alarmin” in surrounding cells. Under inflammatory conditions, pro–IL-1α is cleaved into mature IL-1α that signals either as an extracellular or as a membrane bound molecule. IL-1β is primarily produced by myeloid cells in a precursor form. Upon danger/pathogen-associated molecular pattern (D/PAMP) recognition by pattern recognition receptors and downstream inflammasome-mediated proteolytic activation, pro-IL-1β matures into IL-1β. Secretion of IL-1β can occur via active secretion or via increased cellular permeability once cells go into pyroptosis. IL-1 engagement with IL-1R1/3 leads to downstream pathway activation, which can be prevented by naturally occurring inhibitors like soluble IL-1R1 antagonist (IL-1RN) and antagonistic receptor (IL-1R2, which lacks an intracellular signaling domain). IL-1 pathway inhibiting drugs are depicted. Recombinant human IL-1Ra (anakinra) targets both IL-1α and IL-1β, as does the soluble IL-1R (rilonacept). Neutralizing antibodies selectively target IL-1α (bermekimab) or IL-1β (canakinumab). General and cell-type specific effects of IL-1 signaling pathway activation are indicated. IL-1 pathway targeting compounds are indicated. Right panel depicts the interplay between aging, inflammaging and IL-1 signaling in the BM. Age associated molecular alterations result in the indicated cellular dysfunctions, which converge in inducing inflammaging. Inflammaging leads to multiple tissue alterations that result in D/PAMP production. D/PAMPs are sensed in the BM by myeloid, plasma, and stromal cells leading to increased IL-1 production, which, in turn, is sensed by niche cells and HSCs, triggering niche degradation and myelopoiesis, respectively. This cycle amplifies the local amounts of IL-1, leading in the long term to increased inflammatory burden, further amplifying the initial inflammaging levels.
IL-1 as an inflammaging factor
A conserved feature of the IL-1 pathway is its transient activation status. Fast IL-1 signaling transduction termination is ensured by multiple mechanisms: (1) degradation of internalized IL-1R1; (2) activation of negative-feedback inhibitors of kinase activity downstream of IL-1R1; and (3) expression of ubiquitous soluble IL-1R1 antagonist (IL-1RN), of antagonistic receptors (IL-1R2, lacking an intracellular signaling domain), or of truncated downstream signaling molecules (MYD88 short or IRAK-1c).12 These mechanisms are essential to terminate IL-1–mediated inflammation and prevent its acute off-target damaging effects and associated inflammatory pathologies. Indeed, loss of IL-1RN function in patients with either IL-1RN loss-of-function homozygous mutations or with mutant IL-1R1 with low IL-1RN affinity results in autoinflammatory disease.13,14
A consequence of dysfunctional inflammatory shutdown is the onset of persistent and chronic low-grade inflammatory stimuli developing upon aging in both humans and mice, termed inflammaging. Inflammaging is a condition with a complex etiology that poses a significant risk factor for morbidity and mortality in older individuals (Figure 1, right panel).15 Importantly, HSC functions are highly affected by aging. An aged HSC pool shows reduced homing and repopulating capacity, increased myeloid differentiation bias, accumulated DNA damage and proliferative stress marks, reduced clonal diversity, epigenetic drift, loss of cellular polarity, and decreased autophagy.16 The extent of these alterations that result exclusively from inflammaging signaling remains an active question in the field. However inflammation, when sustained over time, can drive HSC proliferative differentiation into the myeloid lineage at the expense of self-renewal, resulting in decreased repopulating capacity of the HSC pool.2 Concerning the role of IL-1 in inflammaging, it is interesting to note that although its levels are not consistently found elevated in the serum of older individuals, many of its downstream canonical targets, which are considered prototypical inflammaging factors, are indeed elevated in the aging population (eg, IL-6, IL-8, and MCP-117). Furthermore, both IL-1α and IL-β are elevated in the BM of aged mice leading to enhanced/reduced myelopoiesis/lymphopoiesis that results from an IL-1–dependent onset of a transcriptional-driven myeloid differentiation bias in the HSC pool.18,19 Increased local IL-1 production in this context is attributed to multiple BM cell types (macrophages, platelets, dendritic, plasma, and endosteal stromal cells19-23) and results partially from cellular sensing of increased systemic levels of microbiome-derived pathogen-associated molecular patterns (Toll-like receptor 4 and 8 ligands) upon aging.18 These pathogen-associated molecular patterns are derived in large parts from an aging-associated alteration of the intestinal microbiome and increased intestinal permeability.24,25 Importantly, BM inflammation and HSC myeloid differentiation bias are completely abrogated in aged Il1r1 knockout mice, further highlighting the role of IL-1 as an upstream initiator of the inflammaging process in response to increased microbiome-derived signals.18
Effects of IL-1 in HSC biology
IL-1 is implicated in hematopoietic regeneration, prevention of irradiation- or chemotherapy-induced myelosuppression, and protection of neutropenic mice against sepsis.26-28 Part of these effects result from direct sensing of IL-1 by HSCs (here defined as murine lineage−ckit+Sca-1+Flt3−CD48−CD150+ cells), which express multiple members of the IL-1 signaling pathway (Il1r1, Il1rap, Il1r2, Irak1-4, and Myd88), although the protein levels of IL-1R1 are reported as low.22,29 Importantly, how HSCs respond to IL-1 signaling depends on the inflammatory context and on stimuli duration. Indeed, IL-1rn knockout mice, which have sustained increased BM IL-1 levels, show enhanced HSC differentiation toward MPP2s, and sustained myelopoiesis, which associate with upregulated NF-κB and PU.1-dependent transcriptional networks in HSCs.30 Moreover, although both experimentally induced acute and chronic IL-1 exposures (0.5 μg per day per mouse for 1 or 20 days, respectively) lead to HSC proliferation, MPP pool expansion and myelopoiesis through activation of myeloid transcription factor PU.1 (downstream of NF-κB activation), they differentially affect HSC repopulation capacity, causing either no effect (acute) or reducing it (chronic).22,28,29,31,32 Interestingly, the effects of chronic exposure on HSC repopulation capacity are reversible after a withdrawal period, suggesting that sensing of IL-1 by the HSC pool is heterogeneous and highly regulated to prevent excessive proliferative stress and fitness loss.33 Mechanistically, the suppressive effect of IL-1 chronic exposure on HSC repopulation capacity results from a sustained repression of Myc, cell cycle, and protein synthesis genes, which are directly bound by IL-1–induced PU.1.34 This is consistent with previous characterizations of PU.1 as a cell cycle inhibitor and driver of myeloid differentiation by increasing its intracellular accumulation.35,36 Moreover, signal transduction downstream of IL-1R1 (and Toll-like receptors) driving myelopoiesis can be actively shut down in HSCs by E3 ubiquitin ligase speckle-type pox virus and zinc finger (POZ) protein (SPOP)-mediated ubiquitination and subsequent proteasomal degradation of MYD88.37 Thus IL-1 signaling on HSCs seems to have a paradoxical role, inducing, on one hand, HSC proliferation and PU.1-mediated myeloid differentiation but, on the other hand, concomitantly preventing excessive proliferation and pool exhaustion during strong inflammatory conditions by shutting down cell cycle progression via PU.1-mediated gene regulation and potentially by shutting down inflammatory signaling via active MYD88-proteasomal degradation. Interestingly, in vivo levels of IL-1 in the context of aging or in inflammatory diseases (eg, rheumatoid arthritis) seem to have a limited effect on HSCs repopulation capacity but are sufficient to imprint a myeloid differentiating bias, which can be reverted by IL-1 signaling blockage.18,38 These observations reinforce the notion that the amount of IL-1 sensed by HSCs can affect, at a first level, their differentiation potential and, on a secondary and more profound level, reduce their reconstitution capacity and cellular fitness (Figure 2, left panel).
IL-1 signaling pathway as a driver of CH and myeloid malignancy. The left panel depicts the proposed effects of IL-1 on normal and mutant HSCs: the top scheme depicts how WT HSCs respond to IL-1 stimulation. IL-1 exposure levels determine different cellular outcomes. Higher IL-1 exposure results in increased PU.1 levels, decreased proliferation, increased myelopoiesis, and decreased self-renewal. The lower scheme depicts the effects of high IL-1 exposure on Tet2-, Jak2-, and Cepba-mutant HSCs, compared with the effect observed in WT HSCs. “+” indicates a stronger effect in mutant compared with WT. “–” indicates a weaker effect in mutant compared with WT. The central panel depicts how the IL-1 signaling pathway drives CH progression via a self-sustaining feedback loop. Increased IL-1 levels, derived from aged CH-mutant mature myeloid cells, act directly on HSPCs favoring CH-mutant HSPC expansion (circular arrows) and multilineage differentiation (linear arrows) over WT HSPCs. The right upper panel depicts the IL-1 pathway alterations in myeloid malignancies: HSPCs from patients with MDS show increased expression of IL-1β, IL-1R3, and IRAK1. Patients with MPNs have increased levels of IL-1α, IL-1β, and IL-1RN and surface expression of IL-1R1/3 on HSCPs. Patients with CML show increased levels of IL-1β and surface expression of IL-1R3 on HSCPs. Increased levels of IL-1β and IL-1R3 surface expression in blasts associates with poorer survival in patients with AML. Increased IL-1 signaling leads to increased proliferation and survival in HSPCs/blasts from these myeloid malignancies. The right lower panel depicts the nonhematological effects of IL-1 production by CH-mutant myeloid cells: increased IL-1 levels in individuals with CH associate with cardiovascular disease, gout, and potentially with lung and other cancer incidence. FLT3, fms-like tyrosine kinase 3; SCF, stem cell factor.
IL-1 signaling pathway as a driver of CH and myeloid malignancy. The left panel depicts the proposed effects of IL-1 on normal and mutant HSCs: the top scheme depicts how WT HSCs respond to IL-1 stimulation. IL-1 exposure levels determine different cellular outcomes. Higher IL-1 exposure results in increased PU.1 levels, decreased proliferation, increased myelopoiesis, and decreased self-renewal. The lower scheme depicts the effects of high IL-1 exposure on Tet2-, Jak2-, and Cepba-mutant HSCs, compared with the effect observed in WT HSCs. “+” indicates a stronger effect in mutant compared with WT. “–” indicates a weaker effect in mutant compared with WT. The central panel depicts how the IL-1 signaling pathway drives CH progression via a self-sustaining feedback loop. Increased IL-1 levels, derived from aged CH-mutant mature myeloid cells, act directly on HSPCs favoring CH-mutant HSPC expansion (circular arrows) and multilineage differentiation (linear arrows) over WT HSPCs. The right upper panel depicts the IL-1 pathway alterations in myeloid malignancies: HSPCs from patients with MDS show increased expression of IL-1β, IL-1R3, and IRAK1. Patients with MPNs have increased levels of IL-1α, IL-1β, and IL-1RN and surface expression of IL-1R1/3 on HSCPs. Patients with CML show increased levels of IL-1β and surface expression of IL-1R3 on HSCPs. Increased levels of IL-1β and IL-1R3 surface expression in blasts associates with poorer survival in patients with AML. Increased IL-1 signaling leads to increased proliferation and survival in HSPCs/blasts from these myeloid malignancies. The right lower panel depicts the nonhematological effects of IL-1 production by CH-mutant myeloid cells: increased IL-1 levels in individuals with CH associate with cardiovascular disease, gout, and potentially with lung and other cancer incidence. FLT3, fms-like tyrosine kinase 3; SCF, stem cell factor.
Clonal hematopoiesis (CH) and inflammation
A consequence of aging is the cumulative increase in somatic mutational load in long-lived cell populations such as stem cells.39 HSCs are predicted to acquire mutations at a constant rate of 14 to 17 mutations per cell per year in humans and a predicted 8.5-fold higher rate in mice.40-43 Although most mutations are either neutral or are detrimental (and therefore not fixed in the HSC pool), a fraction of the mutations increase HSC fitness compared with their wild-type (WT) counterparts thus leading to relative mutated HSC expansion. Over time, mutant clonal outgrowth leads to the onset of clonal hematopoiesis of indeterminate potential (CHIP; also called aging-related CH or ARCH), defined as the presence of an expanded somatic blood cell clone carrying leukemia driver mutations (eg, mutations in DNMT3A, TET2, ASXL1, TP53, JAK2, and SF3B1 genes) at a variant allele frequency (VAF) of ≥2%, in the absence of hematological malignancy.44,45 CHIP carriers have a ∼13-fold increased risk of developing myeloid hematological malignancies and all-cause mortality, largely due to cardiovascular disease and stroke.46-48 Importantly CHIP-associated comorbidities correlate directly with mutation type, number and higher VAFs,49,50 further highlighting the need to understand the biological drivers of CHIP expansion. Indeed recent advances identified metabolic, inflammatory, and genotoxic pressures as CHIP drivers.51 Moreover, CHIP incidence increases with age, ranging from <1% in individuals aged <40 years and reaching up to 10% to 20% after 70 years of age.52 This likely results from the combined consequences of chronological aging, which increases the risk of driver mutation occurrence, and of physiological aging, which shapes the selective pressures acting on WT and mutant HSCs. Inflammation is a major selective pressure acting during aging (see previous sections) but also in the context of CHIP. Indeed, CHIP carriers have higher IL-6, tumor necrosis factor α (TNFα), IL-1β, and IL-18 serum levels than healthy controls.53 Furthermore, enhanced inflammatory responses have been described in DNMT3A,54,55TET2,56-59 and JAK2 mutant myeloid cells.60 Importantly, multiple studies suggest that CHIP-mutant HSCs, contrary to their WT counterparts, develop molecular adaptations to cope with sustained inflammatory cues present in older individuals with CHIP.2 In the next section, we summarize the main adaptation mechanisms to IL-1 inflammatory pressure observed in TET2, JAK2, and CEBPA mutant HSPCs, in which IL-1–mediated inflammation has been reported as a direct driver of mutant clonal expansion.
IL-1 as a driver of CH: mechanistic adaptations
TET2
The TET2 gene encodes a dioxygenase that promotes DNA demethylation.61TET2 heterozygous loss-of-function mutations are frequent in CHIP (10%-30% prevalence) and in myelodysplastic neoplasms (MDS).62 HSCs with dysfunctional Tet2 show increased self-renewal, repopulation capacity, myeloid differentiation bias, and a predisposition to hematological malignancy.63,64 The role of IL-1 in TET2-mutant CHIP is highlighted by the observation in a large cohort of CHIP carriers that increased IL-1β circulating levels associate significantly and exclusively with TET2-mutant CHIP.53 Moreover, canakinumab (an IL-1β neutralizing antibody) is particularly effective in reducing cardiovascular events in patients carrying hematopoietic TET2 mutations.65 In mouse models, loss of Il1r1 in Tet2−/− HSPCs rescues multiple abnormalities associated with Tet2 deficiency, including increased HSPCs numbers, proinflammatory burden (TNFα and interferon-γ [IFN-γ]), myeloid output,66 and mortality.67 Work from our laboratory further highlighted the dependency of Tet2+/− clonal progression on IL-1 signaling during aging. We observed that Tet2+/− hematopoietic expansion starts 7 to 8 months from the onset of CH and associates with increased inflammaging-derived IL-1α/β, produced mainly by BM Tet2+/− myeloid cells in response to lipopolysaccharide sensing. Importantly, genetic deletion of Il1r1 or pharmacological inhibition of the IL-1–IL-1R1 signaling prevents Tet2+/− clonal expansion during aging. Mechanistically, this associates with increased proliferation, multilineage differentiation, reduced self-renewal and repopulation capacity loss of IL-1α–exposed Tet2+/− HSPCs compared with WT HSPCs.29 More recently, DNA methylation loss in transcription factor binding sites implicated in terminal differentiation (eg, PU.1, ELF4, CEBP, JUN) in WT HSPCs upon IL-1β administration was shown to be attenuated in Tet2−/− HSPCs, which concomitantly upregulate transcriptional signatures associated with self-renewal. These IL-1β–mediated molecular changes in HSPCs favor myelopoiesis and clonal expansion of Tet2−/− hematopoiesis over the WT, and they are partially rescued by IL-1 signaling blockage.67 Although mechanistic discrepancies between the above studies might result from specific experimental setups such as different Tet2 mutational status, IL-1 dosages, or potential context-specific nonredundant roles of IL-1β and IL-1α, these studies converge in proposing IL-1 as a targetable driver of Tet2-mutant hematopoiesis, essentially by favoring a sustained self-renewal and reconstitution capacity of Tet2-mutant HSPCs compared with their WT counterparts (Figure 2, left panel).
JAK2
The JAK2 gene encodes a member of the JAK family of nonreceptor protein tyrosine kinases involved in cytokine receptor signaling. The JAK2V617F variant occurs in 3% of individuals with CHIP68 but becomes the most frequent mutation in patients with myeloproliferative neoplasms (MPNs) for which it is a driver mutation.69-71JAK2V617F reduces the self-renewal capacity of individual HSCs but leads to expansion of downstream progenitor cells.72,73 Circulating levels of IL-1α, IL-1β, and IL-1RN are elevated in mice and patients with JAK2V617F mutant MPN. Furthermore, expression levels of IL-1α, IL-1β, IL-1R1, and IL-1R3 on HSPCs correlate positively with JAK2V617F allele fractions in blood granulocytes. Indeed, in MPN JAK2V617F mouse models, IL-1β produced by mutant hematopoietic cells accelerates the development of BM myelofibrosis, which can be reverted by genetic or pharmacological targeting of the IL-1 pathway. IL-1 pathway inhibition in this context results in a general reduction in BM proinflammatory cytokines, illustrating the role of IL-1 as a primary event for the activation of inflammatory signaling in JAK2-V617F–positive MPN.74 Interestingly, JAK2V617F mutant HSPCs exposed to IL-1β upregulate genes related to myelopoiesis and proliferation. Moreover, IL-1 signaling on BM mesenchymal stromal cells results in increased cytokine production, apoptosis, and collagen production, which are key drivers of fibrosis.75 Despite playing a role in advanced MPN phenotypes, IL-1 is also implicated in earlier stages of MPN transformation. Accordingly, IL-1β secreted by JAK2V617F myelomonocytic cells and megakaryocytes promotes MPN disease initiation by favoring early clonal expansion of JAK2V617F HSCs. Importantly, this effect is attenuated by treatment with an anti–IL-1β antibody.76 Taken together, these studies indicate that IL-1 signaling plays a pathogenic role in JAK2V617F MPN progression by favoring JAK2V617F HSPC expansion in a highly inflammatory environment in which the niche support of WT HSPCs is severely compromised77 (Figure 2, left panel).
CEBPA
CCAAT/enhancer-binding protein α (CEBPA) is a basic leucine zipper transcription factor that regulates myeloid lineage commitment in HSPCs. Although CEBPA is not typically mutated in CHIP, its expression levels are reduced in MDS, MPN, and acute myeloid leukemia (AML) by multiple recurrent genetic alterations.78-83 Loss of Cebpa in mouse HSPCs does not confer a cell-intrinsic competitive advantage except in the context of competitive BM transplantation followed by chronic IL-1β exposure, which results in Cebpa−/− short-term HSC, MPP2, MPP3, and MPP4 expansion and a contraction in mature populations in the blood, consistent with a differentiation block. IL-1β–driven prodifferentiative myeloid programs in MPPs are Cebpa dependent. Strikingly, as observed for IL-1β exposure in Tet2+/− HSPCs,29 stemness gene expression levels are reduced in WT MPPs exposed to IL-1β but maintained in Cebpa−/− MPPs, further endowing the latter with a selective advantage only in the context of a fitness differential, that is, chronic IL-1 exposure.84
Altogether, these data suggest that a key conserved mechanism of mutant HSPC adaptation to chronic IL-1 exposure is a sustained capacity to maintain self-renewal function compared with the WT counterparts, which in the long term favors the expansion of the mutant HSPC pool and eventually promotes the propagation of these mutations into the mature cellular lineages (Figure 2, central panel). A remaining open question is whether this effect is specific to TET2, JAK2, and CEBPA mutant HSPCs or a general effect of IL-1–driven selective pressure on CHIP-mutant clones. Of note, in Dnmt3a-mutant CH in which IFN-γ is the major inflammatory driver of clonal expansion in the context of mycobacterium infection, IL-1 shows a marginal synergetic effect.85 Thus, although experimental evidence is still lacking for other CHIP mutations, we can speculate that IL-1 signaling might synergize to variable degrees with mutation–specific adaptive molecular mechanisms, particularly upon inflammaging onset.
IL-1 dependency in myeloid malignancies: a therapeutic vulnerability
The hypothesis of inflammation as a pathogenic driver in hematopoietic malignancies is edified around epidemiological data, indicating that history of any infectious disease or of autoimmune disease associates with increased risks of myeloid malignancy development.86,87 Since these key association findings, a myriad of studies have established a causative relation between inflammation and hematological malignancy development. Importantly altered IL-1 signaling is consistently reported in different clonal myeloid malignancies,88 suggestive that an early premalignant IL-1 dependency starting at the mutant HSPC level (previous section) might establish a hardwired dependency in advanced malignant states, consequently exposing a potential therapeutic vulnerability. In the following sections, we summarize evidence on the dependency and targetability of the IL-1 pathway in different clonal myeloid malignancies (Figure 2, right upper panel).
CML
Chronic myeloid leukemia (CML) is characterized by an oncogenic BCR::ABL gene rearrangement.89 Increased IL-1β is observed in advanced blast phase compared with chronic phase and healthy controls, and correlates with shorter patient survival.90 Moreover, IL-1β stimulates proliferation of mutant CML HSPCs ex vivo, at concentrations comparable with those observed in CML BM.91 IL-1R3 is upregulated in HSPCs from patients with CML compared with those from controls, and its expression increases with disease progression.92 Furthermore, CML HSPCs can be targeted in vitro and in vivo by anti–IL-1R3 antibodies.93,94 Importantly, IL-1β contributes to CML resistance to BCR-ABL tyrosine kinase inhibitor imatinib by decreasing apoptosis rates through cyclooxygenase 2.95 IL-1β is increased in patients with IFN-α–resistant CML as compared with sensitive patients and healthy controls.96 In CML mouse models, IL-1RN in combination with the tyrosine kinase inhibitor nilotinib reduces numbers of leukemic cells in the blood and BM, and the self-renewal potential of leukemic stem cells, and it increases survival of leukemic mice.97
MPNs
Unlike most patients with polycythemia vera or essential thrombocythemia,98 patients with primary myelofibrosis show increased plasma levels of IL-1β. Moreover, high IL-1β levels in patients with polycythemia vera correlate with fibrotic transformation, poor prognosis, and lower survival.99 Interestingly, IL-1 seems to play a more relevant role within JAK2V617F mutant MPNs (compared with MPNs with other drivers, such as mutated calreticulin). JAK2V617F VAFs in circulating granulocytes correlate positively with circulating levels of IL-1α, IL-1β, and IL-1RN, and with surface expression of IL-1R1/3 on HSCPs. Importantly, in JAK2V617F-mutant MPN mouse models, inhibition of IL-1 signaling reduced leukocytosis and splenomegaly and decreased myelofibrosis, osteosclerosis, and BM neuroglial niche deterioration, thus stalling disease progression.74,75,77
MDS
Dysregulated innate inflammation is a common feature driving MDS disease progression via alterations in both the hematopoietic and stromal compartments.100 Both IL-1β and IL-18 are expressed at high levels in low-risk MDS (LR-MDS) because of increased expression of inflammasome proteins and enhanced pyroptosis in MDS HSPCs, which are triggered by high alarmin S100A9 levels.101 HSPCs from patients with high-risk MDS but not LR-MDS show increased surface levels of IL-1R3,102 and IRAK1 is overexpressed and hyperactivated in MDS HSPCs.103 Moreover, recent molecular profiling of genetically defined LR-MDS samples identified 2 main subgroup phenotypes, defined by low and high levels of IL-1β gene expression. The high IL-1β expressing group contains the genetically defined del(5q) cases and the majority of inflammasome gene expression in this group was attributed to monocytes, thus highlighting the contribution of IL-1–mediated inflammatory milieus to MDS.104
AML
In AML, IL-1β expression by blasts associates positively with poor patient prognosis.105 Moreover, high IL-1R3 expression and/or low IL-1RN expression in AML blasts both associate with reduced patient survival.30,102 In patients with AML undergoing immunotherapy with low-dose IL-2 for remission maintenance, low serum IL-1β levels and high levels of IL-1RN correlate with low leukemic relapse.106 In line with this, IL-1RN deletion from the hematopoietic or stromal compartments promotes preleukemic myelopoiesis.30 Mechanistically, IL-1 drives AML blast proliferation107-109 and apoptosis resistance through phosphoinositide 3-kinases (PI3Ks), IRAK, and ceramidase pathway activation.110,111 Strikingly, an ex vivo functional screen of 94 cytokines, identified IL-1 as capable of expanding blasts in 67% of tested primary AML samples while suppressing normal HSPCs, and IL-1R1 knockdown or inhibition of p38MAPK pathway attenuated in vitro expansion of AML blasts and in vivo disease progression.112 Moreover, IL-1R3 is consistently overexpressed across multiple subtypes of AML compared with healthy HSPCs. Somewhat surprisingly, recent data support that IL-1R3 is not only essential for IL-1 sensing but also physically interacts with, and mediates signaling and proliferative effects through AML-relevant receptor tyrosine kinases fms-like tyrosine kinase 3 (FLT3) and c-KIT (CD117).113,114 Together, these observations have fueled the development of multiple IL-1R3–targeting strategies for AML treatment. Indeed, monoclonal antibodies and chimeric antigen receptor T cells targeting IL-1R3 have shown preclinical efficacy in mouse xenongraft models of AML.115,116 Taken together, these studies highlight an IL-1 signaling dependency in AML blast, which might emerge as a promising therapeutic target.
Future directions
Accumulating knowledge of recent years reveals that after reproductive ages, the sustained production and sensing of IL-1 is generally detrimental for the hematopoietic system by pushing HSPC functional erosion and providing a selective pressure landscape that favors mutant (pre-)malignant clonal HSPC expansion. It now will be critical to fully decipher the mechanistic pathways acting downstream of IL-1 signaling and identify the elements that inhibit healthy and drive mutated HSPCs. A logic consequence of current knowledge is the application of IL-1 signaling pathway inhibition in myeloid neoplasia; however, the precise regimens remain to be tested. Indeed, multiple clinical trials testing the effects of IL-1 pathway inhibitors in myeloid malignancies are already currently underway, which will provide the clinical foundation for further developments (supplemental Table 1, available on the Blood website). Furthermore, it will be essential to determine whether upstream causes of inflammaging (eg, barrier organ leakiness, microbiota composition, and increased cellular senescence) can be tackled to prevent increasing inflammatory burden upon aging. Another key underexplored aspect is how hyperinflammatory mutant myeloid cells, differentiated from expanded mutant HSPCs, affect nonhematopoietic organ function. Remarkably, studies using mouse BM chimeras show that CHIP-mutant proinflammatory monocyte-derived macrophages can drive atherosclerosis via enhanced inflammasome-mediated IL-1β and chemokine secretion.57,117,118 However, whether tissue-infiltrating CHIP-mutant immune cells can affect organ function and local immune regulation, remains to be assessed. Moreover, whether these mutant immune cells contribute to increased nonhematological cancer incidence recently observed in CHIP carriers,119 will need to be determined (Figure 2, right lower panel). Overall, a clear delineation of how IL-1, induced downstream of danger signals, acts as a master regulator of both hematopoietic and nonhematopoietic organ system inflammation-driven aging will be key to open new avenues for the understanding and clinical management of age-related diseases.
Acknowledgments
This work was supported by a Comprehensive Cancer Center Zurich fellowship (F.C.), and the Swiss National Science Foundation (310030_184747/1 and 320030_219676/1 [M.G.M.]).
Authorship
Contribution: F.C. and M.G.M. researched and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Markus G. Manz, Department of Medical Oncology and Hematology, University Hospital Zurich, Raemistr 100, CH-8091 Zurich, Switzerland; email: markus.manz@usz.ch.
References
Author notes
The online version of this article contains a data supplement.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal