Issue Archive
Table of Contents
BLOOD COMMENTARIES
REVIEW ARTICLE
IMiD resistance in multiple myeloma: current understanding of the underpinning biology and clinical impact
Bird and Pawlyn review what we know about resistance to a critical class of drugs for the treatment of multiple myeloma: immunomodulatory imide drugs (IMiDs). This class includes thalidomide, lenalidomide, and pomalidomide, all identified initially by empiric means, as well as the cereblon E3 ligase modulators, such as iberdomide and mezigdomide, identified after the mechanism of action of this class of drug was discovered. The authors highlight mechanisms of resistance that directly interfere with the targeting of cereblon, as well as other mechanisms, and discuss potential options to overcome this resistance.
CLINICAL TRIALS AND OBSERVATIONS
Pembrolizumab in relapsed or refractory primary mediastinal large B-cell lymphoma: final analysis of KEYNOTE-170
Clinical Trials & Observations
Brief Report
Zinzani and colleagues present long-term follow-up data demonstrating that pembrolizumab, a PD-1 inhibitor, is safe and effective in patients with relapsed/refractory primary mediastinal B-cell lymphoma (PMBCL). Of the 22 of 53 patients (41.5%) enrolled achieving response during up to 2 years of therapy, the estimated freedom from progression at 4 years exceeds 80%. Eleven patients (21%) have sustained complete response without further therapy at 4 years of follow-up, providing compelling evidence that some patients with relapsed PMBCL may be cured with checkpoint blockade alone.
IMMUNOBIOLOGY AND IMMUNOTHERAPY
Allogeneic, donor-derived, second-generation, CD19-directed CAR-T cells for the treatment of pediatric relapsed/refractory BCP-ALL
Autologous CD19 chimeric antigen receptor (CAR) T-cell therapy has revolutionized the treatment of pediatric relapsed/refractory B-cell precursor acute lymphoblastic leukemia (R/R BCP-ALL). del Bufalo et al report on their experience using donor-derived CAR T cells in patients unsuitable for an autologous approach. Using T cells from either their prior allogeneic stem cell donor or from a designated donor transduced with a second-generation CD19-CAR, the authors report expansion and persistence of CAR T cells and complete remission without measurable residual disease in 13 patients. Eight remain in remission after a median follow-up of 12 months. These exciting results warrant further prospective study.
LYMPHOID NEOPLASIA
IL-7 receptor expression is frequent in T-cell acute lymphoblastic leukemia and predicts sensitivity to JAK inhibition
Genomic landscape of Down syndrome–associated acute lymphoblastic leukemia
Children with Down syndrome (DS) have a 20 times higher risk of developing acute lymphoblastic leukemia (ALL). Li et al report on comprehensive genomic and transcriptomic analyses of 295 patients with DS-ALL, identifying 15 distinct molecular subtypes, 3 of which have a much higher frequency in DS-ALL compared to ALL in children without DS. For a common specific subset of BCR::ABL1-like ALL, patients with DS have inferior outcomes to children without DS. These data highlight unexpected genomic heterogeneity and reinforce opportunities for use of targeted therapies.
RED CELLS, IRON, AND ERYTHROPOIESIS
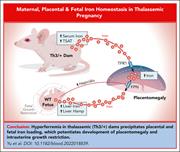
Fetal factors disrupt placental and maternal iron homeostasis in murine β-thalassemia
Iron overload is a common feature in both β-thalassemia major and intermedia due to increased erythropoietic demand, suppression of hepcidin expression, and increased gastrointestinal absorption, with or without transfusions. Yu et al explore the mechanisms of iron homeostasis in β-thalassemia during pregnancy in murine models of these diseases. The authors found that hyperferremia in thalassemic pregnant mice precipitates placental and fetal iron loading, which potentiates development of placental hypertrophy and intrauterine growth restriction. These data help explain some of the increased risk for adverse outcomes of pregnancy in patients with β-thalassemia.
THROMBOSIS AND HEMOSTASIS
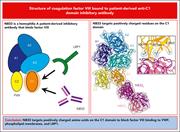
Structure of coagulation factor VIII bound to a patient-derived anti–C1 domain antibody inhibitor
Brief Report
Using single-particle cryo-electron microscopy, Childers and colleagues report on the first structure of factor VIII (FVIII) bound to a recombinant derivative of an anti-C1 domain antibody inhibitor previously isolated from a patient with severe hemophilia A. The inhibitor targets positively charged amino acids on the C1 domain to block FVIII binding to von Willebrand factor, phospholipid membranes, and lipoprotein receptor–related protein 1, which drives FVIII hepatic clearance and antigen presentation in dendritic cells. These data help explain the in vivo consequences of this inhibitor and may assist future engineering of FVIII with a longer half-life due to reduced clearance.
TRANSFUSION MEDICINE
Immunogenicity of quadrivalent meningococcal conjugate vaccine in frequent platelet donors
Clinical Trials & Observations
-
Cover Image
Cover Image
![issue cover]()
Cryo-electron microscopy structure of factor VIII (FVIII) C1 domain (orange) bound to a Fab fragment of an antibody inhibitor (surface). FVIII positively charged residues are depicted as sticks, and the antibody fragment is colored by electrostatic surface potential. See the article by Childers et al on page 197.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals








Pembrolizumab in PMBCL: can it go the distance?
Clinical Trials & Observations