Abstract
Platelet factor 4 (PF4) is a negative regulator of megakaryopoiesis in vitro. We have now examined whether PF4 regulates megakaryopoiesis in vivo by studying PF4 knockout mice and transgenic mice that overexpress human (h) PF4. Steady-state platelet count and thrombocrit in these animals was inversely related to platelet PF4 content. Growth of megakaryocyte colonies was also inversely related to platelet PF4 content. Function-blocking anti-PF4 antibody reversed this inhibition of megakaryocyte colony growth, indicating the importance of local PF4 released from developing megakaryocytes. The effect of megakaryocyte damage and release of PF4 on 5-fluorouracil–induced marrow failure was then examined. Severity of thrombocytopenia and time to recovery of platelet counts were inversely related to initial PF4 content. Recovery was faster and more extensive, especially in PF4-overexpressing mice, after treatment with anti-PF4 blocking antibodies, suggesting a means to limit the duration of such a chemotherapy-induced thrombocytopenia, especially in individuals with high endogenous levels of PF4. We found that approximately 8% of 250 healthy adults have elevated (> 2 times average) platelet PF4 content. These individuals with high levels of platelet PF4 may be especially sensitive to developing thrombocytopenia after bone marrow injury and may benefit from approaches that block the effects of released PF4.
Introduction
Megakaryopoiesis is a complex process that is still not fully understood. Early studies identified thrombopoietin (TPO) as the predominant cytokine responsible for regulating platelet counts. However, many other cytokines have been postulated to participate in regulating megakaryopoiesis by increasing TPO expression in the liver (eg, interleukin-6 [IL-6]), enhancing megakaryocyte chemotaxis (eg, stromal-derived factor-1 [SDF-1]),1 or directly stimulating megakaryocyte development (eg, IL-11).2 A pathway by which megakaryopoiesis is auto–down-regulated has been suggested based on in vitro studies of platelet factor 4 (PF4) and later by studies of other chemokines that are also stored in α-granules, including the related CXC chemokines, neutrophil activating peptide-2 (NAP-2), and IL-8,3,4 and the more distantly related CC chemokines, RANTES (regulated upon activation, normal T-cell–expressed and secreted),5 and MIP-1α (macrophage inflammatory peptide-1α).4,5
PF4 is a 7.8-kDa protein that is produced primarily in megakaryocytes and expressed in platelets as a tetramer, where it comprises a significant portion of the content of α-granules (2.5% on a molar basis).6 The biological role of PF4 is not fully understood. Unlike other chemokines that have clearly defined chemokine receptors, PF4 appears to function by binding with high affinity to glycosaminoglycans on cell surfaces.7–9 PF4 has been proposed to participate in many important biological process based primarily on in vitro studies, including roles in angiogenesis,10 inflammation,11 atherosclerosis,12,13 thrombosis,14–17 and megakaryopoiesis.4,5,18 Studies of infusions of recombinant PF4 in vivo support roles for PF4 in both angiogenesis and thrombosis.19,20
However, there have been little in vivo studies of endogenous platelet PF4 and its role in platelet-mediated physiologic and pathophysiologic processes.20 To address this need, we studied mice that do not express murine (m) PF4 (mPF4−/−) and mice transgenic for human (h) PF4 and that express hPF4 at approximately 6 times the amount found in human platelets (hPF4×6+ mice). We observed that the platelet count among these cohorts of mice appeared to vary inversely with platelet PF4 content. This observation suggests an in vivo role for PF4 in megakaryopoiesis, supporting the previously reported in vitro data. We have now confirmed this observation in inbred mice and extend these studies to demonstrate a role for megakaryocyte PF4 content in colony growth potential and in the recovery from at least some forms of chemotherapy-induced thrombocytopenia. These studies provide a novel approach to augment platelet production in thrombocytopenic conditions, which involve intramedullary megakaryocyte lysis.
Materials and methods
Transgenic mice
All animal lines have been described in detail previously. Briefly, mPF4−/− mice were generated by replacing the entire coding region for mPF4 (1.2 kb) with a 1.8-kb neomycin resistance gene.21 Two transgenic mouse lines that overexpress hPF4 have also previously been described.20 The hPF4×6+ animals used in most of the studies are transgenic for a 14-kb fragment of the human PF4 locus that contains 10.2 kb upstream and 3 kb downstream sequence. Previous analysis of multiple tissues using immunohistochemistry and reverse transcription–polymerase chain reaction (RT-PCR) showed that hPF4 was expressed exclusively in megakaryocytes in these mice,22 and that platelets from hPF4×6+/+ mice have twice the hPF4 levels of hPF4×6+ mice.17 A second hPF4-expressing transgenic mouse line (hPF4×2+) with a 10-kb fragment of the human PF4 locus with 5.4 kb of upstream and 3.8 kb of downstream sequence was also used in several confirmatory studies. The genomic type of all animals was determined by PCR as previously described.20,21 All PF4 variant animals were backcrossed onto a C57BL/6J background for more than10 generations, and comparative studies were done using littermate controls. The mice were housed at the Children's Hospital of Philadelphia animal facility. All procedures were performed after approval by the Institutional Animal Care and Use Committee (IACUC).
Platelet counting and size
Animals were anesthetized and 50 μL of whole blood was obtained by retro-orbital puncture for complete blood counts measured in an automatic cell counter (HEMAVET; Drew Scientific, Dallas, TX) set for mouse parameters. Mean platelet volumes were measured separately on 150 μL of whole blood obtained by retro-orbital puncture and diluted with 150 μL 1.25% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) on the Advia 2120 (Bayer Diagnostics, Tarrytown, NY), which optimized measurement of platelet volume.23–25
Platelet half-life studies
Platelets were isolated from animals that were either wild-type (WT) for PF4 or overexpressed hPF4. Briefly, animals were anesthetized, blood was isolated from the portal vein, and 600 μL of blood was placed in acid-citrate-dextrose (ACD) buffer and centrifuged at 200g for 10 minutes at room temperature to prepare platelet-rich plasma (PRP).26 The platelets were then pelleted by centrifugation at 800g for 20 minutes at room temperature. This pellet was resuspended and washed once in a Tyrode buffer (134 mM NaCl, 3 mM KCl, 0.3 mM NaH2PO4, 2 mM MgCl2, 5 mM HEPES, 5 mM glucose, 1 mg/mL albumin, 5 U/mL apyrase, and 1 mM EGTA). Platelets were then labeled with the Vybrant CFDA SE Cell Tracer kit (Molecular Probes, Eugene, OR) using a 10-μM concentration of CFDA SE (carboxy fluorescein diacetate, succinimidyl ester) and incubated at 37°C for 15 minutes. Platelets were then washed with Tyrode buffer and upon resuspension were immediately injected intravenously through the tail vein into recipient animals. Platelets isolated from 4 animals were pooled and transfused into 1 recipient WT C57BL/6J or hPF4×6+ animal, respectively, such that each animal received a dose of approximately 1 × 1012 platelets. Platelet counts were measured on the HEMAVET in blood obtained by retro-orbital puncture of recipient animals at approximately 12-hour intervals. Cellular fluorescence was used as a marker for the transfused platelets, and the percentage of platelets expressing this marker was measured using flow cytometry.
Measurement of serum TPO levels
Serum was prepared from whole-blood samples obtained by retro-orbital puncture of anesthetized animals. Blood was allowed to clot at 37°C for 30 minutes. Next specimens underwent centrifugation at 2000g for 20 minutes at room temperature. Serum was removed and stored in 25 μL aliquots at −20°C to allow for confirmatory testing on each specimen. Samples were analyzed using the commercially available murine TPO (mTPO) enzyme-linked immunosorbent assay (ELISA) kit, Quantikine (R&D Systems, Minneapolis, MN). Each specimen was run in duplicate.
In vitro BMMNC culture studies
Bone marrow from the tibias and femurs of 6- to 12-week old male mice was isolated and used for in vitro cell culture as previously described.27 Briefly, Iscove modified Dulbecco medium (IMDM; Invitrogen, Carlsbad, CA) without modification was used to flush the marrow cavity of bones harvested from killed mice, and the cells were then passed over a 100-μm nylon filter (BD Biosciences, San Jose, CA). After centrifugation at 1800 rpm, the cell pellet was resuspended in 5 mL ammonium chloride solution (StemCell Technologies, Vancouver, BC, Canada) and incubated at room temperature for 10 minutes to lyse the red cells. After a second centrifugation step, the pelleted cells were resuspended in IMDM + 1% penicillin-streptomycin (Invitrogen) and cultured at 37°C for 18 to 24 hours. Cells were maintained continuously in serum-free conditions. Nonadherent cells were counted and resuspended in base media (IMDM, 3 mg/mL NaHC03, 500 μg/mL β-mercaptoethanol) at a concentration of 1 × 109 cells/L. These cells were then cultured in a murine, serum-free bone marrow mononuclear cell (BMMNC) culture system based on previous methodology described for human bone marrow.28,29 Briefly, cells were suspended in media containing insulin (20 μg/mL), cholesterol (6.16 ng/mL), stem cell factor (10 ng/mL), and recombinant mTPO (50 ng/mL) and mIL-3 (10 ng/mL) (both from R&D Systems) to enhance megakaryocyte differentiation. Media also contained 30 μg/mL partially saturated transferrin, 0.01% BSA, L-asparagine (10 μM), and CaCl2 (37 μg/mL). Clot formation was done by addition of 0.2 U/mL of thrombin and fibrinogen (0.2% wt/vol). No supportive stromal cells were included in the cultures.
In some studies, recombinant murine or human PF4 (20 μg/mL), prepared as previously described,30 was added to the final culture. Briefly, affinity chromatography using a HiTrap Heparin (GE Healthcare, Piscataway, NJ) high-performance affinity column was used to purify the supernatant of lysed BL21DE30 pLysS bacterial cells expressing WT murine or human recombinant PF4 from a pT7–7 plasmid containing the appropriate cDNA. These proteins were further purified by fast protein liquid chromatography (FPLC) using a Resource RPC FPLC column (Amersham Pharmacia Biotech, Piscataway, NJ). Purity was assessed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining. An immunoblot after electrotransfer to polyvinylidenedifluoride was used to confirm the identity of the protein. Primary antibodies were rabbit polyclonal antihuman or antimurine PF4 antibodies (both prepared by PeproTech, Rocky Hill, NJ). Detection was performed with horseradish peroxidase (HRP)–conjugated swine antirabbit antibody (DAKO, Carpinteria, CA) using an electrochemiluminescence (ECL) kit (Perkin Elmer Life Sciences, Shelton, CT). In some colony-formation studies, 25 μg/mL of 1 of the 2 rabbit anti-PF4 antibodies or rabbit pretreatment IgG control (PeproTech) was included to block the effects of free PF4.
Plates were cultured at 37°C under high humidity for 7 to 10 days. Afterward, the plates were removed from the incubator and dried by application of 1:3 acetone-methanol mix for 10 minutes, followed by a wash with PBS, and then dried overnight. The following day, the plates were stained with a rat polyclonal antimouse CD41 antibody (BD Pharmingen, San Diego, CA) and labeled using FITC-conjugated anti-rat IgG antibody (Sigma, St Louis, MO). The plates were analyzed by counting the total number of colonies per plate as well as the number of colonies showing positive fluorescence for megakaryocytes. A ratio of megakaryocyte-containing colonies to total colonies was then calculated for each plate, and results from duplicate plates were averaged. All colonies on each plate were counted (between 30-100 colonies per plate per experiment). The ratios were then normalized to untreated, WT control plates from that experiment to decrease interexperiment variability.
Human CD34+ cell cultures and measurement of released PF4
Human CD34+ cord blood cells (StemCell Technologies) were cultured in liquid serum-free media as previously described.28 Briefly, 4 × 105 purified human CD34+ cells were cultured in media consisting of IMDM with 10 mg/mL BSA (Sigma), 740 μg/mL partially saturated transferrin (Sigma), 10 μg/mL human insulin (Sigma), 40 μg/mL low-density lipoprotein (Calbiochem, San Diego, CA), 2 mM L-glutamine (Fisher Scientific, Hampton, NH) and 100 ng/mL recombinant human TPO (R&D Systems). Cells were incubated at 37°C, and the media was replaced with fresh media on days 4, 7, 11, and 14 of culture. Media (1:10 dilution) was assayed for hPF4 by quantitative ELISA using the Asserachrom PF4 Enzyme Immunoassay of PF4 (Diagnostica Stago, Parsippany, NJ). Recombinant hPF4 of known concentrations were assayed simultaneously to allow for conversion of international units per milliliter to milligrams per milliliter. All specimens were analyzed in duplicate.
In vivo studies of platelet counts and response to chemotherapy
WT, mPF4−/−, or hPF4×6+ female mice aged 8 to 12 weeks were injected intraperitoneally with 180 mg/kg of 5-fluorouracil (5-FU) on day 0. Every 1 to 3 days, complete blood counts were measured on whole blood obtained by retro-orbital puncture. Some animals were also injected with F(ab′)2 fragments prepared using a commercially available kit (ImmunoPure F(ab′)2 kit; Pierce Biotechnology, Rockford, IL) from either rabbit polyclonal anti-human or anti-murine PF4 IgG or control rabbit IgG. Injections were given via the tail vein 15 minutes prior to the 5-FU. An identical dose was administered on day 3 after injection of 5-FU. The total dose of F(ab′)2 fragments was 50 to 100 mg/kg. Additional experiments were performed injecting 200 U heparin sulfate or an equivalent volume of phosphate-buffered sodium solution intradermally daily on days 0 to 7 after injection of 5-FU or protamine sulfate (0.5 mg/kg) intravenously on daily on days 0 to 5 after 5-FU.
Expression of platelet PF4 levels in an adult population
Residual samples of whole blood from healthy adult blood donors from the American Red Cross were processed anonymously after institutional review board approval from the Children's Hospital of Philadelphia. Informed consent was obtained in accordance with the Declaration of Helsinki. Platelets were enumerated in the HEMAVET cell counter. Serum was generated by adding 20 μL of 1 M CaCl2 to 1 mL of whole blood for 1 hour at 37°C. Specimens were centrifuged at 13 000 rpm for 5 minutes, then the serum was removed and diluted 1:1000 in PBS and hPF4 was measured by ELISA (Asserachrom PF4 Enzyme Immunoassay of PF4).31 The mean of duplicate serum measurements was determined, and PF4 even was expressed relative to the platelet count.
Statistics
Differences between groups were compared using the Student t test. Statistical analyses were performed using Microsoft Excel (Redmond, WA). Results were considered significant at P values of .05 or less.
Results
Inverse relationship between platelet count/thrombocrit and platelet PF4 content
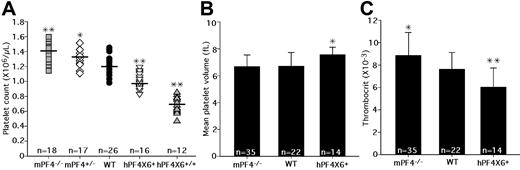
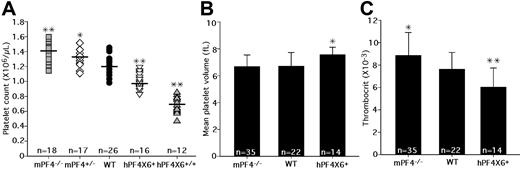
In a preliminary analysis of mPF4−/− mice and hPF4×6+ transgenic mice, we had reported an inverse relationship between platelet count and PF4 content.20 To validate these findings, we backcrossed the animals for more than 10 generations, and measured the platelet counts in mPF4−/−, mPF4+/−, WT littermates, hPF4×6+, and hPF4×6+/+ mice. We found a consistent, inverse relationship between peripheral blood platelet count and platelet PF4 content (Figure 1A). WT C57Bl/6J animals have a baseline platelet count of 1.22 plus or minus 0.12 × 109/L. Platelet counts in PF4-deficient mice were higher than in WT littermates, varying from 1.33 plus or minus 0.17 × 109/L in mPF4+/− mice (P < .02 compared with WT) to 1.40 plus or minus 0.12 × 109/L in mPF4−/− mice (P < .001 compared with WT), which represents a 15% increase over that of the WT littermate controls (Figure 1A). hPF4-overexpressing animals had lower platelet counts. The hemizygous hPF4×6+ mice had platelet counts of 0.98 plus or minus 0.08 × 109/L (P < .001 compared with WT), and homozygous hPF4×6+/+ mice had baseline platelet counts of 0.70 plus or minus 0.06 × 109/L (P < .001 compared with WT), a 47% decrease compared with WT littermate controls (Figure 1A). In addition, animals from a separate founder line with a different transgenic hPF4 DNA fragment, containing less upstream sequence but more downstream sequence,21 and which contained a 2-fold excess of hPF4 compared with human controls, (hPF4×2+),21 also had a decrease in platelet counts to 1.04 plus or minus 0.13 × 109/L compared with their WT littermates at 1.24 plus or minus 0.12 × 109/L (P = .005; data not shown), showing that the observed decrease was not limited to a specific transgenic line and suggesting a dose response. Other cell counts examined in these animals, including hemoglobin, white blood cell count (WBC), and absolute neutrophil count (ANC), were normal (data not shown).
Platelet counts, MPVs, and thrombocrits in transgenic animals. (A) The graph shows the distribution of platelet counts in the different transgenic animals, from left to right: mPF4−/−, mPF4+/−, WT, hPF4×6+, and hPF4×6+/+. Mean value is indicated by horizontal bar for each phenotype. n indicates number of animals studied. *P < .02 and **P < .001 compared with WT littermates. (B) MPVs for each animal type are shown + 1 SD. n indicates number of animals per arm. *P < .003 compared with WT littermates. (C) The mean thrombocrit in each animal type is shown + 1 SD. n indicates number of animals per arm. *P < .03 and **P < .005 compared with WT littermates.
Platelet counts, MPVs, and thrombocrits in transgenic animals. (A) The graph shows the distribution of platelet counts in the different transgenic animals, from left to right: mPF4−/−, mPF4+/−, WT, hPF4×6+, and hPF4×6+/+. Mean value is indicated by horizontal bar for each phenotype. n indicates number of animals studied. *P < .02 and **P < .001 compared with WT littermates. (B) MPVs for each animal type are shown + 1 SD. n indicates number of animals per arm. *P < .003 compared with WT littermates. (C) The mean thrombocrit in each animal type is shown + 1 SD. n indicates number of animals per arm. *P < .03 and **P < .005 compared with WT littermates.
We then studied the relationship between platelet count and total platelet mass as determined by thrombocrit (platelet count times the mean platelet volume [MPV]). Unlike platelet counts, there was no difference in the MPVs between mPF4−/− mice and WT littermates (Figure 1B), and the thrombocrit in mPF4−/− animals was significantly increased by 16% compared with WT littermates (P = .03; Figure 1C). MPV was 13% higher in the hPF4×6+ mice compared with WT littermate controls (P < .004; Figure 1B), but the thrombocrit was still 26% lower in hemizygous hPF4×6+ mice compared with WT littermates (P < .002; Figure 1C). Thus, thrombocrit, as a measurement of total body platelet mass, as well as platelet counts, varied inversely with platelet PF4 content.
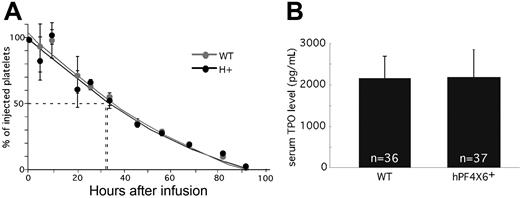
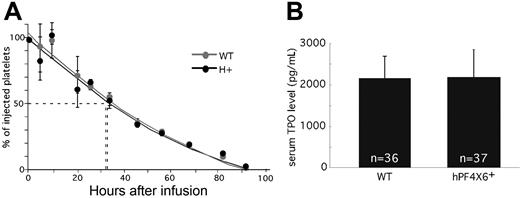
We then asked whether the decreased platelet counts and thrombocrit in the hPF4×6+ mice were due to a shortened platelet half-life. To address this possibility, we compared the half-life of platelets from hPF4×6+ and WT littermates that had been CFDA SE–labeled and transfused back into either hPF4×6+ or WT animals, respectively (Figure 2A). The use of CFDA SE labeling allowed for long-term identification of the transfused platelets. Initial studies done to establish the appropriate concentration of tracer documented that there was no decay of signal even after 7 days after labeling (data not shown; but see Park et al30 ). The half-life of both hPF4×6+ and WT littermate platelets was 37 hours. This suggests that the difference in platelet count is due to an effect of PF4 on platelet production rather than platelet survival. In addition, we wanted to show that this difference in platelet count was not related to peripheral suppression of TPO levels by PF4. To examine this, we compared TPO levels in serum (Figure 2B) and plasma (data not shown) between the hPF4×6+ mice and WT littermate controls. TPO levels were not different between overexpressing and WT mice, so that overexpression of PF4 does not inhibit steady-state circulating TPO levels.
Half-life of injected platelets and serum TPO levels. (A) The percentage remaining of injected CFDA SE–labeled platelets at each time from either hPF4×6+ (●) or WT ( ) mice injected into animals of the same background as measured by flow cytometry is shown. Means values ± 1 SD are shown. n = 3 per arm. (B) Serum PF4 levels in hPF4×6+ animals and WT littermates. n indicates the number of specimens. Data shown as average + 1 SD. Each specimen was analyzed in duplicate.
) mice injected into animals of the same background as measured by flow cytometry is shown. Means values ± 1 SD are shown. n = 3 per arm. (B) Serum PF4 levels in hPF4×6+ animals and WT littermates. n indicates the number of specimens. Data shown as average + 1 SD. Each specimen was analyzed in duplicate.
Half-life of injected platelets and serum TPO levels. (A) The percentage remaining of injected CFDA SE–labeled platelets at each time from either hPF4×6+ (●) or WT ( ) mice injected into animals of the same background as measured by flow cytometry is shown. Means values ± 1 SD are shown. n = 3 per arm. (B) Serum PF4 levels in hPF4×6+ animals and WT littermates. n indicates the number of specimens. Data shown as average + 1 SD. Each specimen was analyzed in duplicate.
) mice injected into animals of the same background as measured by flow cytometry is shown. Means values ± 1 SD are shown. n = 3 per arm. (B) Serum PF4 levels in hPF4×6+ animals and WT littermates. n indicates the number of specimens. Data shown as average + 1 SD. Each specimen was analyzed in duplicate.
In vitro studies of the effect of PF4 and blocking anti-PF4 antibodies
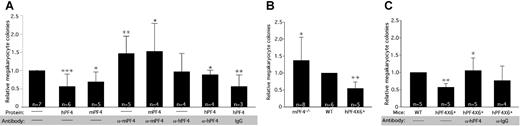
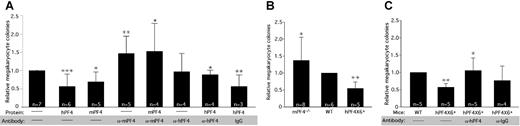
Previous studies have shown that supplementing in vitro cultures of marrow progenitor cells with 25 μg/mL hPF4 decreased the number of megakaryocyte-containing colonies by approximately 50%.18 We asked if mPF4 also inhibited formation of megakaryocyte-containing colonies. As shown in Figure 3A, addition of 20 μg/mL of recombinant mPF4 to WT hematopoietic cells inhibited megakaryocyte-containing colonies by more than 40% (P < .009), comparable to the effect of hPF4 (Figure 3A). Even though plasma levels of PF4 are relatively low,32 recent data suggest that at the site of platelet activation, PF4 levels may be higher than 200 μg/mL;33 thus, these levels of 20 μg/mL of PF4 are within the physiologic range. Addition of polyclonal anti-mPF4 antibody specifically reversed the inhibitory effect of supplemental mPF4, as did an anti-hPF4 antibody in the presence of recombinant hPF4 (Figure 3A). These data indicate that inhibition of megakaryocyte-containing colony formation by PF4 is not species-specific, and that the 2 polyclonal anti-PF4 antibodies can block PF4's inhibitory effect on megakaryopoiesis.
In vitro studies of the effect of PF4 and blocking antibodies. (A) The effect of recombinant human or mouse PF4 on numbers of megakaryocyte colonies from WT marrow and the ability of species-specific polyclonal antibodies to block this effect. Relative means of megakaryocyte colonies are shown + 1 SD *P < .02, **P < .005, and ***P < .001 each compared with untreated WT. The star indicates P < .03 compared with WT + PF4 of same species. (B) Relative means + 1 SD of megakaryocyte colonies compared with WT controls for mPF4−/− and hPF4×6+ mice are shown. *P < .04 compared with WT, **P < .001 compared with WT. (C) The effect of adding anti-hPF4 antibodies to bone marrow from hPF4×6+ animals. *P < .009 compared with hPF4×6+ baseline. **P < .001 compared with WT. Data were normalized to WT in each experiment in all panels to control for interexperiment differences in total number of colonies obtained.
In vitro studies of the effect of PF4 and blocking antibodies. (A) The effect of recombinant human or mouse PF4 on numbers of megakaryocyte colonies from WT marrow and the ability of species-specific polyclonal antibodies to block this effect. Relative means of megakaryocyte colonies are shown + 1 SD *P < .02, **P < .005, and ***P < .001 each compared with untreated WT. The star indicates P < .03 compared with WT + PF4 of same species. (B) Relative means + 1 SD of megakaryocyte colonies compared with WT controls for mPF4−/− and hPF4×6+ mice are shown. *P < .04 compared with WT, **P < .001 compared with WT. (C) The effect of adding anti-hPF4 antibodies to bone marrow from hPF4×6+ animals. *P < .009 compared with hPF4×6+ baseline. **P < .001 compared with WT. Data were normalized to WT in each experiment in all panels to control for interexperiment differences in total number of colonies obtained.
We then examined marrow progenitors derived from mPF4−/−, WT, and hPF4×6+ mice to study the effect of endogenous PF4 stores on the formation of megakaryocyte-containing colonies. We hypothesized that release of stored PF4 would act as a negative autocrine regulator of megakaryocyte colony formation. Megakaryocyte colony number was inversely related to PF4 content. Bone marrow from mPF4−/− mice developed 40% more megakaryocyte colonies than WT controls (P < .04), while hPF4×6+-overexpressing marrow developed 50% fewer megakaryocyte-containing colonies (P < .001; Figure 3B). Addition of the anti-mPF4 antibody in cultures of mPF4−/− bone marrow cells was able to reverse the effect of exogenous PF4, but had no effect on colony formation when used alone (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Polyclonal anti-hPF4 antibody increased colony formation from hPF4-overexpressing marrow while control preimmune IgG did not (Figure 3C). Indeed, anti-hPF4 antibody actually increased the number of megakaryocyte-containing colonies cultured from hPF4×6+ bone marrow to 30% higher than the number of colonies formed from WT marrow, although this did not reach statistical significance (Figure 3C).
Release of PF4 from human CD34+ cells upon differentiation into megakaryocytes in serum-free media
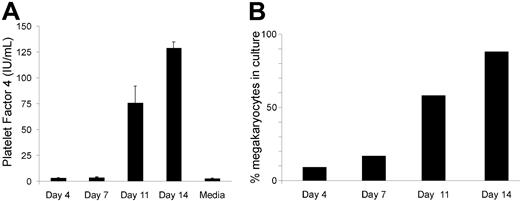
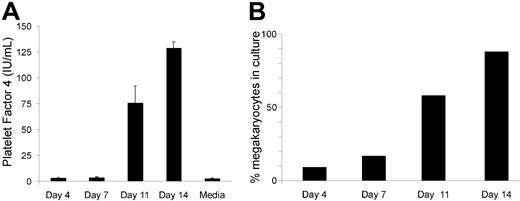
Because there is an inherent difference in megakaryocyte colony numbers between WT and hPF4×6+ mice in vitro without addition of serum or other sources of exogenous PF4 and without any stromal cell support, the effect of anti-PF4 shown in Figure 3 suggests that inhibition of megakaryocyte colony formation is mediated by PF4 released from developing megakaryocytes. To support the idea that PF4 is released from developing megakaryocytes, we measured PF4 levels in the serum-free media of human CD34+ as they mature into megakaryocytes. As can be seen in Figure 4A, human PF4 levels are undetectable at day 0 and day 7, consistent with our attempts to remove any PF4 in our cultures. On day 11, there is an increase in the levels of PF4 in the media (without addition of exogenous PF4 to the media), suggesting release by cells in culture. This level of PF4 increases by day 14 to the limit of detection by the assay (about 100 ng/mL). At this point, the cultures consist of more than 80% megakaryocytes by flow cytometry (Figure 4B).
Concentrations of hPF4 released into the media during megakaryocyte differentiation of human CD34+ cells. (A) hPF4 level changes in the media of human CD34+ cells grown in TPO only in serum-free media. n = 2 separate studies done in duplicate. Mean + 1 SD shown. (B) Analysis of percentage of CD41+-derived megakaryocytes at the same day points of culture as in panel A for 1 of the studies.
Concentrations of hPF4 released into the media during megakaryocyte differentiation of human CD34+ cells. (A) hPF4 level changes in the media of human CD34+ cells grown in TPO only in serum-free media. n = 2 separate studies done in duplicate. Mean + 1 SD shown. (B) Analysis of percentage of CD41+-derived megakaryocytes at the same day points of culture as in panel A for 1 of the studies.
Effect of PF4 on platelet recovery in vivo after chemotherapy
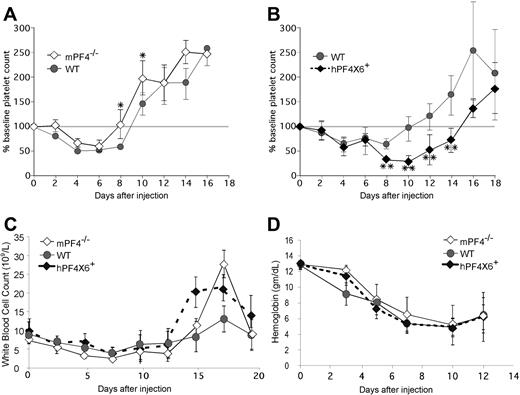
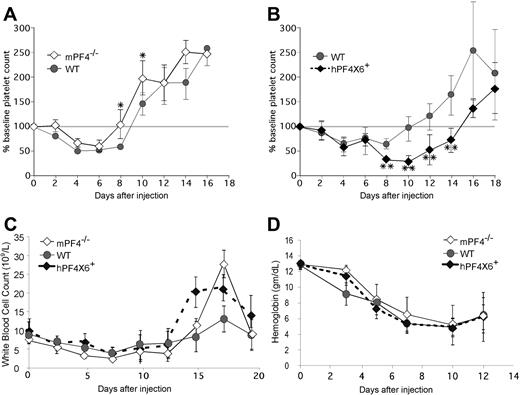
Based on these findings, we hypothesized that the duration of thrombocytopenia after chemotherapy might be protracted by release of PF4 from chemotherapy-injured megakaryocytes. To test this hypothesis, mPF4−/−, WT, and hPF4×6+ mice were injected with the cytolytic drug 5-FU and followed for the recovery in platelet counts. We observed an inverse relationship between PF4 content and duration of thrombocytopenia (Figure 5). mPF4−/− mice recovered almost 2 days faster compared with WT controls (9.1 ± 1 vs 10.8 ± 1.3 days; P = .003; Figure 5A). Hemizygous hPF4×6+ mice took more than 4 days longer than the WT controls (15.3 ± 1.7 days; P = .001; Figure 5B). Recovery was defined as return to more than 80% of baseline because above that level, no animal dropped their platelet counts again, whereas below that level of recovery, counts might spontaneously decrease again (data not shown). The hPF4×6+ mice had an approximately 45% lower nadir platelet count than WT controls (0.29 ± 0.2 × 109/L vs 0.61 ± 0.2 × 109/L; P < .001). The difference was also significant when expressed as the relative decrease in platelet count from baseline (nadir platelet count of 23% ± 15% of baseline in the hPF4×6+ mice vs 50% ± 7% of baseline for WT littermates; P < .001). Similar results were seen after injection of 5-FU in the other hPF4-expressing line (hPF4 × 2+), which had a mean time to platelet count recovery of 14.5 plus or minus 1.6 days versus 10.3 plus or minus 1.5 days in WT littermates (P = .005) with a nadir platelet count of 0.3 plus or minus 0.1 × 109/L versus 0.7 plus or minus 0.2 × 109/L for WT littermates (P = .009; data not shown). In the hPF4×6+ studies, initial blood counts and recovery of WBC (Figure 5C), ANC (data not shown), and hemoglobin levels (Figure 5D) did not vary in a manner consistent with endogenous PF4 levels.
The in vivo effect of PF4 on platelet count recovery after injection of 5-FU. (A) Mean relative change in platelet count ± 1 SD in WT (•) and mPF4−/− (◇) animals after injection of 5-FU shown as percentage of baseline platelet count. (B) Same as in panel A, but for WT mice controls ({9I}) and hPF4×6+ mice (♦). (C) The same as panels A and B, but for total WBC. (D) The same as panel A, but for hemoglobin. All studies were done in triplicate with 5 to 6 mice per arm (graphs represent data from 15-18 animals per arm). *P < .03 compared with WT. **P < .003 compared with WT. Grey horizontal bar denotes 100% of baseline platelet count and is drawn to allow for easier discrimination of recovery.
The in vivo effect of PF4 on platelet count recovery after injection of 5-FU. (A) Mean relative change in platelet count ± 1 SD in WT (•) and mPF4−/− (◇) animals after injection of 5-FU shown as percentage of baseline platelet count. (B) Same as in panel A, but for WT mice controls ({9I}) and hPF4×6+ mice (♦). (C) The same as panels A and B, but for total WBC. (D) The same as panel A, but for hemoglobin. All studies were done in triplicate with 5 to 6 mice per arm (graphs represent data from 15-18 animals per arm). *P < .03 compared with WT. **P < .003 compared with WT. Grey horizontal bar denotes 100% of baseline platelet count and is drawn to allow for easier discrimination of recovery.
We then tested several approaches to block the effect of the released PF4 on the platelet count following 5-FU–induced transient marrow failure. Concurrent with the 5-FU infusion, we began a 7-day course of subcutaneous heparin (1000 U/kg daily) to bind PF4 and displace it from cell-surface glycosaminoglycans,34 or 5 days of protamine sulfate (10 mg/kg daily) to neutralize PF4's activity.35,36 However, neither heparin nor protamine sulfate altered the severity or duration of the thrombocytopenia in either WT or hPF4×6+ mice (data not shown). Since the effect of these agents are short-lived,37,38 we turned to the blocking antimurine and antihuman PF4 antibodies, which might be expected to exert their effects continuously for at least several days after infusion.39 Unexpectedly, we noted an accelerated fall in platelet counts during the first week after combined therapy with 5-FU and blocking anti-PF4 antibody (data not shown), a result that we posited might have been mediated through the interaction of IgG with the mice's FcγRs. To address this possibility, we repeated these studies using F(ab′)2 fragments derived from these antibodies or isoimmune control.
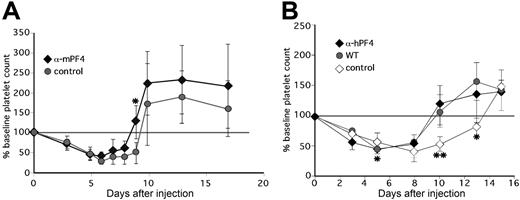
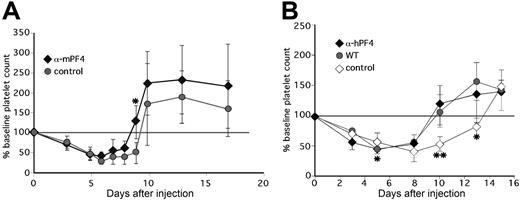
Injection of anti-mPF4 antibody F(ab′)2 fragments in WT animals concurrent with 5-FU and again 3 days later accelerated recovery of the platelet count (Figure 6A). Approximately 33% of the anti-mPF4–treated animals had platelet count recovery by day 8, whereas none of the animals given control F(ab′)2 fragments showed a similar recovery of the platelet count before day 10 (P < .003). The average time to recovery to baseline platelet count was 8.7 plus and minus 1.3 days versus 10.5 plus or minus 1.0 days (P < .003) for animals receiving anti-mPF4 therapy versus controls. The platelet counts in hPF4×6+ mice given anti-hPF4 F(ab′)2 improved to that of concurrently studied WT controls, while hPF4×6+ mice given isoimmune control had no improvement (Figure 6B). The mean time to recovery to baseline in the F(ab′)2 anti-hPF4–treated group was 9.7 plus or minus 1.0 days versus 13.0 plus or minus 1.0 days in the group given control F(ab′)2 (P = .001), and was similar to the 10.2 plus or minus 2.2 days seen in concurrently studied WT littermates. These data clearly demonstrate that the same anti-murine and anti-human PF4 antibodies that block the inhibitory effect of PF4 on megakaryocyte colony formation in vitro reduced the duration of thrombocytopenia after chemotherapy in vivo.
The in vivo effect of anti-PF4 antibodies. (A) Same as in Figure 4, but for change in platelet count in WT animals injected with either F(ab′)2 fragments prepared from IgG from preimmunization serum (•) or from polyclonal anti-mPF4 antibodies (♦). *P ≤ .05. (B) Same as in panel A, but for change in platelet count from baseline of WT animals (•) versus littermate hPF4×6+ animals injected with either F(ab′)2 fragments from either anti-hPF4 (♦) or preimmune IgG (◇). For panels A and B, *P < .03 and **P < .003 comparing mice treated with preimmune IgG to the mice treated with anti-PF4 antibody. Each point in this figure represents the mean of 6 to 12 animals plus or minus 1 SD.
The in vivo effect of anti-PF4 antibodies. (A) Same as in Figure 4, but for change in platelet count in WT animals injected with either F(ab′)2 fragments prepared from IgG from preimmunization serum (•) or from polyclonal anti-mPF4 antibodies (♦). *P ≤ .05. (B) Same as in panel A, but for change in platelet count from baseline of WT animals (•) versus littermate hPF4×6+ animals injected with either F(ab′)2 fragments from either anti-hPF4 (♦) or preimmune IgG (◇). For panels A and B, *P < .03 and **P < .003 comparing mice treated with preimmune IgG to the mice treated with anti-PF4 antibody. Each point in this figure represents the mean of 6 to 12 animals plus or minus 1 SD.
Variation in human platelet-PF4 expression
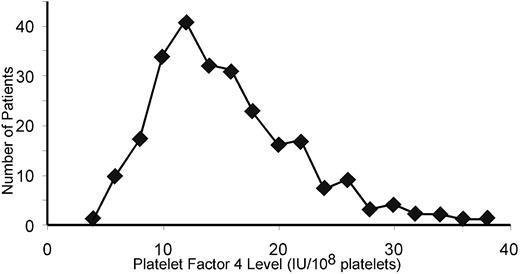
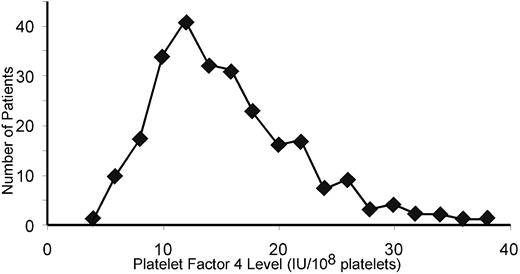
These in vitro and in vivo data suggest that the inhibitory effect of PF4 on megakaryopoiesis is inversely related to platelet-PF4 content. Based on these observations, we began to analyze the variation in platelet-PF4 content in humans by examining the PF4 per platelet of 250 healthy human blood donors. PF4 expression followed a bell-shaped curve with a tail to the right (Figure 7). The mean platelet PF4 level was 15.3 IU/108 platelets. Approximately 8% of the population sampled had levels of PF4 per platelet that were more than twice the mean of the entire population.
Variation in platelet PF4 content in the human population. The distribution of PF4 content in platelets in 250 healthy human blood donors is shown.
Variation in platelet PF4 content in the human population. The distribution of PF4 content in platelets in 250 healthy human blood donors is shown.
Discussion
While the TPO/c-mpl axis is the predominant regulator of platelet count (reviewed in40 ), it is clear that other factors regulate megakaryocyte maturation and platelet release. Previous results from in vitro hematopoietic colony assays showed that supplemental PF4 inhibits megakaryocyte colony formation.5 In this study, we show that there is an inverse relationship between both platelet count and thrombocrit compared with platelet PF4 content in genetically modified mice. This negative effect of endogenous PF4 is enhanced following marrow damage by chemotherapy, and blocking anti-PF4 antibodies accelerated platelet recovery.
The data presented are consistent with a model of megakaryopoiesis in which PF4 is released from developing megakaryocytes, and this PF4 is sufficient to limit further megakaryopoiesis. The number of megakaryocyte-containing colonies formed in vitro in serum-free media, but with TPO present, varied inversely with PF4 expression. The inclusion of anti-PF4 antibodies in the culture system enhanced megakaryocyte development and also blocked the inhibitory effect of added recombinant PF4. Further megakaryocytes derived from cultured human CD34+ cells release significant amounts of PF4 in serum-free media. In contrast to published studies suggesting that PF4's effect on megakaryopoiesis may be by suppressing TPO production by bone marrow stromal cells,41 our in vitro data suggest that this effect is intrinsic to the developing megakaryocyte progenitors, as light-density cells were cultured without stromal cell support. The mechanism by which PF4 is released during megakaryopoiesis requires further study and may occur continuously and/or during platelet release. Additional studies are needed to definitively identify not only the target cell for PF4 action, but also the receptor involved in this process.
The inverse relationship between platelet counts and platelet PF4 levels were notable even with smaller changes in PF4 content such as between mPF4+/− and hPF4×2+ mice and their respective WT littermates. These platelet count changes among the various genetically modified mice are notable even though all the animals studied had an intact TPO/c-mpl axis. Published studies of the role of SDF-1 in megakaryopoiesis did not show any differences in platelet counts until SDF-1 was given to animals who had a defect in the TPO/c-mpl axis.42 Our studies did not find a difference in the steady-state TPO levels in the hPF4×6+ mice, but TPO biology differs from the biology of the related cytokine erythropoietin, where the kidney is the overwhelming source and levels of renal erythropoietin expression vary inversely to the degree of anemia.43 In healthy individuals without inflammation, TPO expressed by the liver is constant.44 In addition, significant amounts of TPO are produced by stromal cells as well, and the biology of this pool of TPO is not well understood.45 Circulating TPO levels clearly do not reflect the degree of marrow megakaryocyte activity in clinically relevant situations such as idiopathic thrombocytopenic purpura (ITP).46
It has been suggested that PF4 also inhibits the formation of other hematopoietic lineages based on in vitro colony formation.16,47 Our in vivo data show that PF4-deficient and PF4-overexpressing mice have similar hemoglobins and ANCs after 5-FU compared with WT controls. The initial drop in WBC after 5-FU chemotherapy are also not different, but at the end of 2 weeks, the overshoot recovery in both the PF4-deficient and –overexpressing mice was greater than the controls. Whether there is a biological basis to this greater recovery remains to be determined.
Other chemokines are expressed during megakaryopoiesis and stored in platelet α-granules, including the closely related CXC chemokine NAP-2, which is also largely restricted in its expression to developing megakaryocytes.48 More widely expressed CXC chemokines such as IL-8 and CC chemokines, such as RANTES and MIP-1α, are also expressed by megakaryocytes and stored in platelet α-granules.49 In vitro colony formation studies suggest that these chemokines inhibit megakaryopoiesis and do so at even lower concentrations than PF4. Preliminary analysis of mice that are deficient in NAP-2 or overexpress its human homolog by 2-fold do not show any steady-state hematologic differences from WT littermates, nor is there a delay in platelet count recovery in these animals after 5-FU (data not shown). Reports on murine knockout models for deficiencies of the murine IL-8 homolog,50 RANTES,50 and MIP-1α51,52 do not comment on a decrease in platelet counts, nor are there reports of platelet count changes in these animals after being challenged with cytolytic therapies. Whether any of the other chemokines has biologically relevant in vivo effects on platelet count remains to be examined to define whether inhibition of additional chemokines will enhance the effect of PF4 blockade on megakaryopoiesis.
A previous study suggested that PF4 may reduce the sensitivity of hematopoietic progenitors to cytotoxic agents.53 In that study, the only effects on megakaryopoiesis were seen 8 days after administration of PF4, even though PF4 has a half-life of only 25 minutes.54 Our data do not support a proposed protective effect of PF4 in cytolytic therapy. In fact, our results are the opposite of what would have been expected for PF4 being a protective agent of hematopoietic progenitors, but are consistent with in vitro studies presented in this paper and others,4,5,18,54 showing that PF4 directly inhibits the generation of mature megakaryocytes.
In summary, we have shown that PF4 down-regulates platelet counts in vivo through an effect on megakaryocyte progenitors. Even WT developing megakaryocytes release sufficient PF4 to act as a negative paracrine regulator in a lineage-specific manner. In a murine model of 5-FU–induced marrow injury, the severity and duration of the suppression of platelet production appears to vary inversely with PF4 expression in the developing megakaryocytes. Administration of anti-PF4 blocking antibodies reverses this inhibition and promotes platelet count recovery. Based on the variability of PF4 levels in a normal human population, we believe that these findings may have important clinical implications for the care of patients with thrombocytopenias involving intramedullary megakaryocyte lysis. Further investigation of these observations may lead to novel therapeutic approaches to treat or prevent thrombocytopenia in a number of clinical settings.
An Inside Blood analysis of this article appears at the front of this article.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the American Red Cross for their supply of human blood donor blood for the PF4 variability studies. We also thank Suresh Shelat of the Division of Pathology and Laboratory Medicine at the Children's Hospital of Philadelphia for his assistance with the platelet volume studies using the Advia machine, and Douglas B. Cines of the University of Pennsylvania for critical reading of this manuscript.
This work was supported in part by funding from the American Heart Association (M.P.L.) and the NIH (PO1 HL40387 to M.P.).
National Institutes of Health
Authorship
Contribution: M.P.L. was the primary investigator and carried out most of the experiments and developed more of the experimental direction with time. She also wrote the initial draft and many of the revisions. L.R. performed the human studies in Figure 7 and was helpful in many other studies and data interpretation. M.B. performed the experiments with human cord blood–derived CD34+ cells in Figure 4. M.C.S.-V. assisted in planning and performing the human CD34+ cell experiments and assisted in manuscript revision. M.A.K. performed many of the initial studies and taught M.P.L. the techniques involved, and also assisted in data interpretation and manuscript revision. M.P. is the senior author who provided overall scientific direction, data interpretation, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mortimer Poncz, The Children's Hospital of Philadelphia, 3615 Civic Center Blvd, ARC, Rm 317, Philadelphia, PA 19104; e-mail: poncz@email.chop.edu.


 ) mice injected into animals of the same background as measured by flow cytometry is shown. Means values ± 1 SD are shown. n = 3 per arm. (B) Serum PF4 levels in hPF4×6+ animals and WT littermates. n indicates the number of specimens. Data shown as average + 1 SD. Each specimen was analyzed in duplicate.
) mice injected into animals of the same background as measured by flow cytometry is shown. Means values ± 1 SD are shown. n = 3 per arm. (B) Serum PF4 levels in hPF4×6+ animals and WT littermates. n indicates the number of specimens. Data shown as average + 1 SD. Each specimen was analyzed in duplicate.