Abstract
Platelet P-selectin plays important roles in inflammation and contributes to thrombosis and hemostasis. Although it has been reported that von Willebrand factor (VWF) affects P-selectin expression on endothelial cells, little information is available regarding regulation of platelet P-selectin expression. Here, we first observed that P-selectin expression was significantly decreased on platelets of fibrinogen and VWF double-deficient mice. Subsequently, we identified this was due to fibrinogen deficiency. Impaired P-selectin expression on fibrinogen-deficient platelets was further confirmed in human hypofibrinogenemic patients. We demonstrated that this impairment is unlikely due to excessive P-selectin shedding, deficient fibrinogen-mediated cell surface P-selectin binding, or impaired platelet granule release, but rather is due to decreased platelet P-selectin content. Fibrinogen transfusion completely recovered this impairment in fibrinogen-deficient (Fg−/−) mice, and engagement of the C-terminus of the fibrinogen γ chain with β3 integrin was required for this process. Furthermore, Fg−/− platelets significantly increased P-selectin expression following transfusion into β3 integrin–deficient mice and when cultured with fibrinogen. These data suggest fibrinogen may play important roles in inflammation, thrombosis, and hemostasis via enhancement of platelet P-selectin expression. Since human fibrinogen levels vary significantly in normal and diseased populations, P-selectin as an activation marker on platelets should be used with caution.
Introduction
Platelets play a crucial role in both hemostasis and thrombosis. They also contribute to inflammation, including immune-mediated inflammation,1-3 and the development of atherosclerosis.4 At the site of vascular injury, particularly at high shear stress, the binding of the platelet GPIb complex to von Willebrand factor (VWF) on the injured vessel wall initiates platelet tethering and subsequent adhesion. Platelet aggregation is then mediated by interaction between platelet β3 integrin and fibrinogen (Fg), although Fg-independent platelet aggregation can also occur.5-7 During platelet activation and degranulation, P-selectin translocates to the platelet surface. Through interaction with sulfatides8 and the GPIb complex,9 P-selectin may also contribute to the stabilization of platelet aggregates. Although Fg, VWF, and P-selectin are all important molecules that support hemostasis and thrombosis and are all involved in inflammation, their mutual impact on these processes is poorly understood. It is completely unknown whether Fg or VWF affects P-selectin expression on the platelet surface.
P-selectin is a transmembrane protein belonging to the selectin family of leukocyte adhesion receptors. It is synthesized by megakaryocytes and endothelial cells and is subsequently stored in the α-granules of platelets10 and the Weibel-Palade bodies of endothelial cells.11 Upon agonist stimulation of platelets or endothelial cells, the granules may fuse with the plasma membrane allowing P-selectin to translocate to the cell surface, where it may be shed via cleavage by a currently unknown sheddase(s).12,13 It has been reported that VWF affects P-selectin translocation to the endothelial cell surface,14 but it is unclear whether VWF and/or Fg affect P-selectin translocation in platelets. In addition, although platelets contain a significant amount of mRNA and are able to synthesize proteins de novo15,16 (eg cytokines17 and β3 integrin18 ), it is unknown whether P-selectin synthesis can occur in platelets. It is also unclear whether platelet P-selectin content can be regulated by platelet receptor “outside-in” signaling or by α-granule biogenesis in megakaryocytes.
In this study, we first observed that P-selectin expression was significantly decreased on thrombin-activated platelets from Fg/VWF double-deficient (Fg/VWF−/−) mice. We subsequently demonstrated that this was specifically due to deficiency of Fg, but not VWF. The impaired P-selectin expression on Fg-deficient (Fg−/−) platelets was further confirmed in a human patient with severe hypofibrinogenemia and his heterozygous parents. We found that the platelet P-selectin content was lower in Fg−/− platelets, and that Fg transfusion into Fg−/− mice completely restored platelet intracellular and cell surface P-selectin expression. We demonstrated that β3 integrin engagement with the C-terminus of the Fg γ chain is required to maintain normal levels of platelet intracellular P-selectin. The possible mechanisms of how Fg may enhance intracellular P-selectin levels are discussed.
Methods
Mice
Fg/VWF−/− mice were described previously5,6 and have been backcrossed onto a C57BL/6J background to generate Fg+/+, Fg+/−, and Fg−/− littermates and VWF−/− mice. Mice with a mutation in the C-terminus of the Fg γ chain (FgγΔ5) have also been described previously.19,20 β3 integrin gene–deficient (β3−/−) mice and GPIbα gene–deficient (GPIbα−/−) mice were kindly provided by Dr Richard O. Hynes (Massachusetts Institute of Technology, Cambridge, MA) and Drs Jerry Ware and Zaverio M. Ruggeri (The Scripps Research Institute, La Jolla, CA), respectively. Genotypes of all experimental animals were confirmed by polymerase chain reaction (PCR) analysis of tissue DNA. All mice were housed in the research vivarium of St Michael's Hospital in Toronto and the Children's Hospital Research Foundation in Cincinnati. The experimental procedures were approved by the respective Animal Care Committees of each participating institution.
Human subjects
The patient with severe hypofibrinogenemia (homozygous) is a 31-year-old male born from heterozygous parents of a consanguineous marriage. The patient has an Fg β chain mutation with a plasma Fg level of 0.05 μM (approximately 0.5% of the normal level). His parents both have Fg levels approximately 50% of normal. Their clinical manifestation and genetic diagnosis have been reported previously.21,22 Healthy blood donors were used as controls. Informed consent was obtained for each subject in accordance with the Declaration of Helsinki. This study was approved by the institutional review board at Anhui Provincial Hospital.
Reagents
Thrombin was purchased from Haematologic Technologies and Diagnostica Stago for mouse and human studies, respectively. Thrombin receptor-activating peptides (TRAP, AYPGKF-NH2, and SFLLRN-NH2) were purchased from Peptides International. Goat antimouse P-selectin (CD62P) and goat anti–human actin were purchased from Santa Cruz Biotechnology. Polyclonal rabbit anti–human P-selectin (BD Biosciences), polyclonal rabbit anti–human platelet factor 4 (PF4; Chemicon International), mouse monoclonal anti–human β-actin (clone AC-15; Sigma-Aldrich Canada), and mouse monoclonal anti–human thrombospondin-1 (TSP-1; clone D4.6; Lab Vision) were purchased from the indicated companies. Mouse antihuman vinculin, alkaline phosphatase (AP)–conjugated rabbit anti–goat IgG, AP-conjugated goat anti–mouse IgG, and human plasma Fg were purchased from Sigma-Aldrich Canada. Rabbit antihuman fibrinogen was purchased from American Diagnostica and AP-conjugated goat anti–rabbit IgG was purchased from Assay Designs. Bovine serum albumin (BSA), NP-40, EDTA, PIPES, 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium (BCIP/NBT), and phorbol myristyl acetate (PMA) were purchased from Sigma-Aldrich Canada. GM6001 and A23187 were purchased from Calbiochem.
Blood collection and preparation of gel-filtered platelets
Mice (6-8 weeks old) from each genotype studied were anesthetized with 2.5% tribromoethanol (0.015 mL/g) and bled via the retro-orbital plexus using a heparin-coated glass capillary tube. Blood was collected into a tube containing ACD (38 mM citric acid, 75 mM trisodium citrate, 100 mM dextrose) or EDTA (10% of final volume). Platelet-rich plasma (PRP) was obtained by centrifugation at 300g for 7 minutes. Platelets were then isolated from the PRP using a Sepharose 2B column. The blood collection and platelet preparation of human subjects have been described previously.21,22
Detection of P-selectin expression on mouse platelets by flow cytometry
Resting or activated (thrombin 1 U/mL TRAP [AYPGKF-NH2] 0.5 mM to 2 mM) platelets were incubated with fluorescein isothiocyanate (FITC)–conjugated rat antimouse CD62P (BD Biosciences) for 30 minutes at room temperature in the dark. Phosphate-buffered saline (PBS, 0.5 mL) was added to the sample immediately before acquisition. All samples were analyzed via flow cytometry using calibrated FACScan flow cytometer (Becton Dickinson). Ten thousand events per sample were acquired; light scatter and fluorescence channels were set at a logarithmic gain. The platelet population was analyzed for mean fluorescence intensity. For each piece of experiment, all samples for comparison were acquired at the same settings.
Detection of P-selectin expression on human platelets by flow cytometry
Gel-filtered platelets were activated with 1.5 U/mL thrombin (Diagnostica Stago) or 100 μM TRAP (SFLLRN-NH2) in the presence of 1 mM Ca2+ for 3 minutes. The platelets were fixed with 1% paraformaldehyde at 4°C overnight before examination. To label P-selectin, paraformaldehyde-fixed, activated platelets were incubated with phycoerythrin (PE)–conjugated mouse antihuman CD62P (BD Biosciences) for 1 hour at room temperature before being analyzed by flow cytometry on a FACScan flow cytometer (EPICS XL-MCL; Beckman Coulter).
Detection of P-selectin, fibrinogen, TSP-1, and PF4 by Western blot
Gel-filtered mouse and human platelets (4 × 107 to 1 × 108) were lysed, separated by SDS–polyacrylamide gel electrophoresis (PAGE), transferred onto polyvinylidene fluoride (PVDF) membrane, and probed with polyclonal goat antimouse P-selectin (or rabbit antihuman P-selectin) and goat antihuman actin antibodies. For some immunoblots (ie, PF4 and TSP-1), washed mouse platelet lysates were prepared by differential centrifugation (PRP: 300g for 7 minutes; wash: 1500g for 5 minutes) as previously described for human platelets.23 The rabbit antihuman Fg (cross-reacts with mouse Fg) antibody was used to detect plasma and platelet Fg. BCIP/NBT was added following incubation with AP-conjugated anti–goat or anti–rabbit IgG to visualize the protein bands. Mouse PF4 and TSP-1 immunoblots and human P-selectin and TSP-1 immunoblots from platelet lysates were detected via chemiluminescence (ECL) as previously described.23
Detection of P-selectin by enzyme-linked immunosorbent assay
Plasma and platelet P-selectin was measured by Quantikine Mouse P-selectin enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems). Blood samples were collected into EDTA-coated cuvettes and were centrifuged at 1400g for 10 minutes to remove the cells and collect the plasma. Platelets (107) were lysed in 1% NP-40 buffer. Plasma or platelet samples (50 μL) were diluted and incubated in coated wells for 2 hours at room temperature. After washing, a conjugated anti–P-selectin antibody was added to the plates and incubated for 2 hours. Substrate was added for 30 minutes after 5 washes to determine activity. The reaction was terminated and the samples were read at 540 nm in an ELISA plate reader (Bio-Tek Instruments).
Plasma fibrinogen transfusion
Human Fg (Sigma-Aldrich Canada) used for transfusion contains approximately 60% protein (> 91% of protein is clottable), 15% sodium citrate, and 25% sodium chloride. The Fg was injected intravenously to Fg−/− and β3−/− (negative control) mice at a dose of 0.1 mg/g. For transfused Fg−/− mice, plasma was taken at 30 minutes, 1 hour, 6 hours, 1 day, 2 days, and 4 days after transfusion and mice were sacrificed on days 1, 2, and 4 after Fg transfusion to obtain platelets. For transfused β3−/− mice, platelets were obtained 2 days after transfusion.
Quantitative morphometric comparisons of α-granule contents in platelets with electron microscopy
Murine blood was collected into a tube containing 3.8% sodium citrate (1/9, vol/vol). PRP was centrifuged at 800g for 10 minutes, fixed for 1 hour with 2.5% glutaraldehyde in PBS, pH 7.4, at 4°C, and processed for electron microscopy as previously described.23 Thin sections were examined with a JEOL JEM-1230 electron microscope (JEOL Ltd) where digital images were acquired with a side-mounted Advanced Microsystems Techniques (AMT) Advantage HR CCD camera (Advanced Microsystems Techniques). The quantitative morphometric comparisons were performed in 50 platelets per mouse and 2 mice in each group were quantitated according to previously described methods.24
Quantitation of platelet P-selectin mRNA using reverse transcription–PCR and quantitative real-time PCR
Total platelet RNA was extracted from WT, Fg−/−, and β3−/− mouse platelets using RNeasy Plus Mini Kit (QIAGEN), and cDNA was synthesized using QuantiTect Reverse Transcription Kit (QIAGEN). PCR amplification was performed using primers for mouse P-selectin and β-actin, which have been described.25 Real-time PCR was performed using P-selectin primers (5′-CCTGGCAAGTGGAATGATGA-3′ and 5′-GAGCAGGTATAGCTCCCAA-3′) and β-actin primers (5′-GTGGGCCGCTCTAGGCACCAA-3′ and 5′-GCCATGTTCAATGGGGTAC-3′) with the SYBR Green PCR Master Kit and the StepOne Real-Time PCR System (Applied Biosystems) based on the manufacturer's protocol.
Platelet transfusion and protein detection by flow cytometry
Gel-filtered platelets (2 × 108) from Fg−/− mice (β3-integrin positive) were transfused intravenously into β3−/− mice (Fg positive) and Fg−/− mice (negative control). Transfused mice were sacrificed 2 days after transfusion to isolate platelets via gel filtration. Flow cytometry was performed with either resting, TRAP-, or thrombin-activated platelets, which were double stained using PE-conjugated hamster anti–mouse CD61 (integrin β3 subunit; BD Biosciences) and either FITC-conjugated rat anti–mouse CD62P or FITC-conjugated goat anti–mouse Fg (Nordic Immunological Laboratories). β3 integrin–positive Fg−/− platelets were gated and 5000 β3 integrin–positive events per sample were acquired.
Detection of P-selectin levels in platelets incubated with fibrinogen in vitro
Blood was collected from Fg−/− mice into 1% (wt/vol) EDTA-PBS, pH 7.4, and platelets were isolated by differential centrifugation (PRP: 300g for 15 minutes; wash: 1050g for 15 minutes with 50 ng/mL prostaglandin E1 [Sigma-Aldrich Canada]). Platelets were resuspended in RPMI 1640 media (Invitrogen Canada) and supplemented with human Fg (2 mg/mL) or an equal volume of saline (vehicle control) and incubated at 37°C overnight with gentle rotation. Following incubation, platelets (4 × 107) were lysed and Western blotting was performed as described in “Detection of P-selectin, fibrinogen, TSP-1 and PF4 by Western blot” using horseradish peroxidase–conjugated donkey anti–goat IgG (Santa Cruz Biotechnology) and detected via ECL.
Statistical analysis
Data are presented as mean plus or minus SEM. Statistical significance was assessed by unpaired Student t test.
Results
Fibrinogen, but not VWF, deficiency impaired platelet surface P-selectin expression
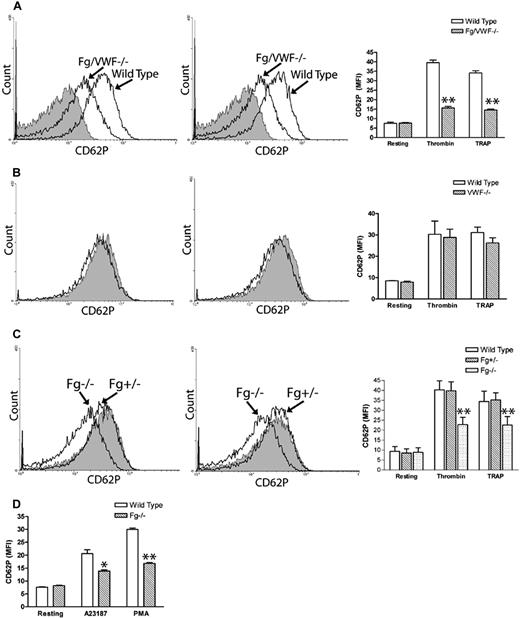
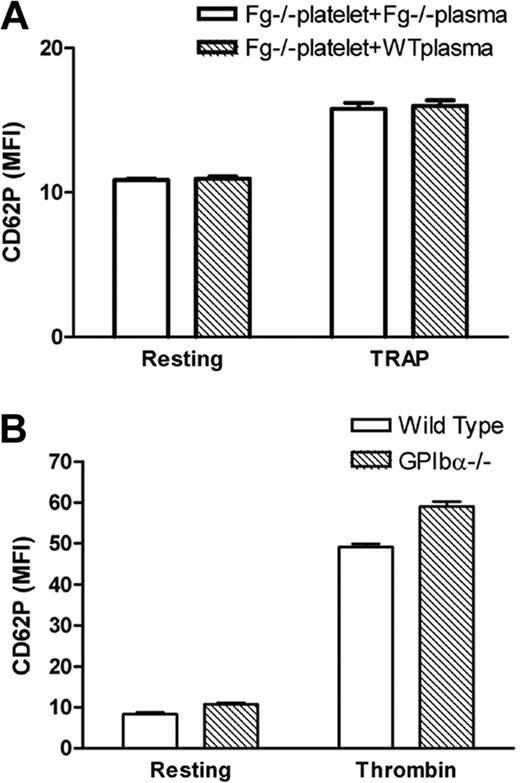
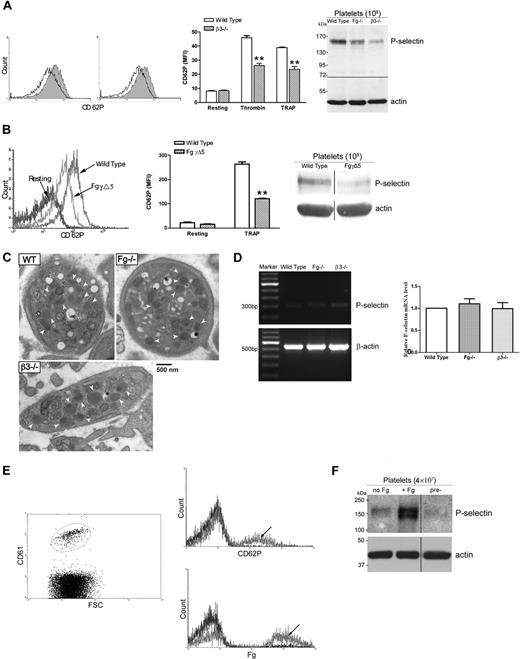
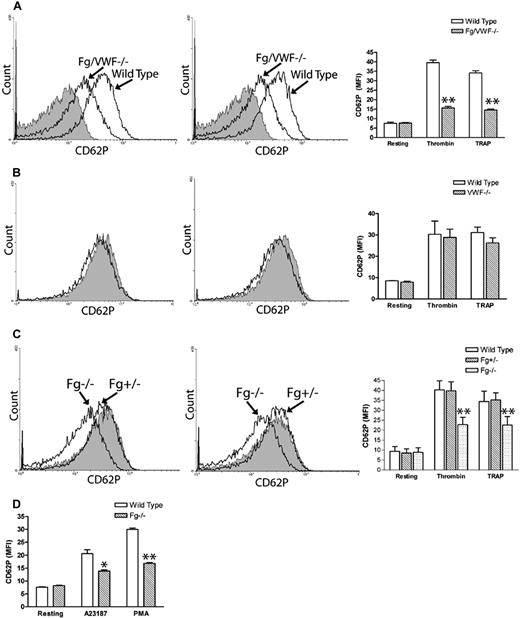
While investigating Fg- and VWF-independent thrombosis, we observed a significant decrease in P-selectin expression on thrombin- and TRAP-activated Fg/VWF double-deficient (Fg/VWF−/−) platelets. The mean fluorescence intensity (MFI) decreased approximately 50% to 60% compared with wild-type controls (Figure 1A). Since VWF supports P-selectin expression on activated endothelial cells,14 we examined whether the observed phenomenon resulted from VWF deficiency. We generated VWF−/− mice by backcrossing Fg/VWF−/− mice to the C57BL/6J background, and observed no significant difference in platelet P-selectin expression after platelet activation, although a minor decrease in expression cannot be completely excluded (Figure 1B). The same results were obtained using commercial VWF−/− C57BL/6J mice from The Jackson Laboratory (data not shown). Subsequently, we identified that the decreased P-selectin expression was likely due to Fg deficiency. To confirm this result, we generated Fg+/+, Fg+/−, and Fg−/− littermates to minimize genetic background differences. As shown in Figure 1C, a significant decrease in P-selectin expression on activated Fg−/− platelets was detected compared with wild-type littermates. Impaired P-selectin expression on the Fg−/− platelet surface was also observed when the agonist A23187 (10 μM) or PMA (100 μM) was used to induce platelet activation (Figure 1D). These results demonstrate, for the first time, that Fg plays a role in platelet P-selectin surface expression, which markedly differs from endothelial cells in which VWF affects cell surface P-selectin expression.14
Fibrinogen, but not VWF, deficiency impaired P-selectin expression on the platelet surface. Gel-filtered platelets were incubated with either thrombin (left panels) or TRAP (middle panels), and P-selectin expression on the platelet surface was analyzed via flow cytometry (A-C). Right panels and Figure 1D indicate mean fluorescence intensity (MFI) ± SEM. (A) A significant decrease in platelet surface P-selectin expression was observed on thrombin- or TRAP-activated Fg/VWF−/− platelets compared with wild-type controls (arrow indicated); n = 3 (**P < .001). No significant difference in P-selectin expression was observed on resting Fg/VWF−/− platelets compared with wild type (shaded). (B) No significant difference in platelet P-selectin expression was observed on VWF−/− platelets (black) compared with wild type (shaded); n = 3, P = .85 (thrombin), P = .24 (TRAP). (C) A significant decrease in P-selectin expression on the surface of activated Fg−/− platelets (arrow indicated) was observed compared with wild-type littermate controls (shaded); n = 3 (**P < .001). No significant difference was observed between Fg+/− and wild type; n = 3 (P > .05). (D) A significant decrease in P-selectin expression on the surface of A23187- and PMA- activated Fg−/− platelets was observed compared with wild type; n = 3 (*P < .05, **P < .01, respectively).
Fibrinogen, but not VWF, deficiency impaired P-selectin expression on the platelet surface. Gel-filtered platelets were incubated with either thrombin (left panels) or TRAP (middle panels), and P-selectin expression on the platelet surface was analyzed via flow cytometry (A-C). Right panels and Figure 1D indicate mean fluorescence intensity (MFI) ± SEM. (A) A significant decrease in platelet surface P-selectin expression was observed on thrombin- or TRAP-activated Fg/VWF−/− platelets compared with wild-type controls (arrow indicated); n = 3 (**P < .001). No significant difference in P-selectin expression was observed on resting Fg/VWF−/− platelets compared with wild type (shaded). (B) No significant difference in platelet P-selectin expression was observed on VWF−/− platelets (black) compared with wild type (shaded); n = 3, P = .85 (thrombin), P = .24 (TRAP). (C) A significant decrease in P-selectin expression on the surface of activated Fg−/− platelets (arrow indicated) was observed compared with wild-type littermate controls (shaded); n = 3 (**P < .001). No significant difference was observed between Fg+/− and wild type; n = 3 (P > .05). (D) A significant decrease in P-selectin expression on the surface of A23187- and PMA- activated Fg−/− platelets was observed compared with wild type; n = 3 (*P < .05, **P < .01, respectively).
Impaired P-selectin expression on fibrinogen-deficient human platelets
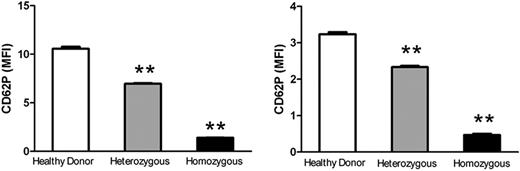
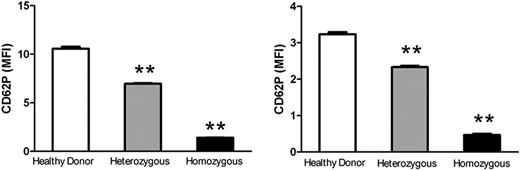
To investigate the observed phenomenon in a clinical setting, we examined P-selectin expression on platelets, using flow cytometry, from a patient with severe hypofibrinogenemia, his heterozygous parents, and healthy donors. Consistent with our mouse data, a significant decrease in the patient's platelet surface P-selectin expression was observed after thrombin or TRAP activation (Figure 2), in which the MFI of the patient's platelet P-selectin was 6.4- or 7.5-fold lower than that of the healthy donors (P < .001; Figure 2), and 4.6- or 4.9-fold lower than that of his heterozygous parents (P < .001; Figure 2). Even the heterozygous parents, with 50% of normal plasma Fg levels, had significantly lower platelet surface P-selectin expression compared with healthy controls (P < .001; Figure 2). This further demonstrated that Fg plays a significant role in platelet P-selectin expression, and suggests that it may affect this process in a dose-dependent manner.
Impaired P-selectin expression on fibrinogen-deficient human platelets. Cell surface P-selectin expression was detected on thrombin- or TRAP-activated healthy control, heterozygous, and homozygous platelets using flow cytometry. A significant decrease in P-selectin expression on the surface of heterozygous and homozygous platelets was observed compared with healthy controls after thrombin treatment (left panel, MFI ± SEM, **P < .001) or TRAP treatment (right panel, MFI ± SEM, **P < .001). The experiments were repeated at least 3 times.
Impaired P-selectin expression on fibrinogen-deficient human platelets. Cell surface P-selectin expression was detected on thrombin- or TRAP-activated healthy control, heterozygous, and homozygous platelets using flow cytometry. A significant decrease in P-selectin expression on the surface of heterozygous and homozygous platelets was observed compared with healthy controls after thrombin treatment (left panel, MFI ± SEM, **P < .001) or TRAP treatment (right panel, MFI ± SEM, **P < .001). The experiments were repeated at least 3 times.
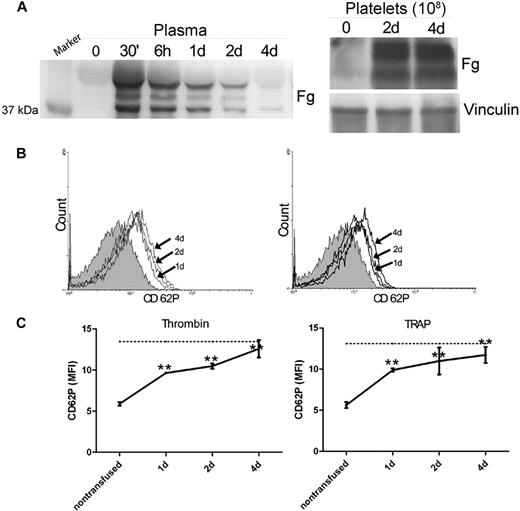
Fibrinogen transfusion restored platelet surface P-selectin expression in Fg−/− mice
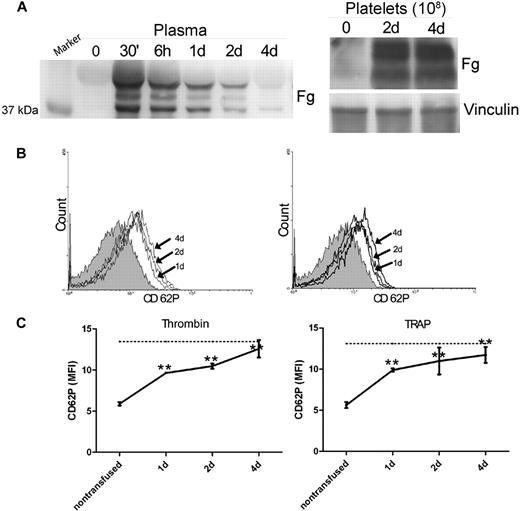
To confirm the role of Fg in platelet P-selectin expression, we transfused plasma Fg into Fg−/− mice. The Fg used for transfusion does not contain any detectable P-selectin via immunoblot (data not shown). Thirty minutes after transfusion, we observed significant levels of Fg in the blood circulation, which gradually decreased but were detectable until 4 days after transfusion (Figure 3A). The Fg−/− mice were sacrificed 1, 2, or 4 days after transfusion to obtain platelets. Significant Fg was stored in the platelets of Fg−/− mice after Fg transfusion and persisted until 4 days after transfusion (Figure 3A). Notably, on day 4 when the Fg in plasma was markedly reduced, significant amounts of Fg were still detected in platelets, indicating that the intraplatelet pool of Fg is distinct from the plasma pool. Interestingly, a significant increase in P-selectin expression on the surface of Fg−/− platelets was observed 1 day after transfusion, and the level of expression continued to increase 2 days and 4 days after transfusion (Figure 3B). P-selectin expression reached levels similar to (∼ 80%) wild-type littermate controls at 2 days after transfusion. Thus, transfusion of plasma Fg into Fg−/− mice was able to completely restore the platelet surface P-selectin expression to normal levels (Figure 3C).
Fibrinogen transfusion recovered platelet surface P-selectin in Fg−/− mice. (A) Plasma Fg was determined by Western blot 30 minutes (30′), 6 hours (6h), 1 day (1d), 2 days (2d), and 4 days (4d) after transfusion. Thirty minutes after transfusion, significant plasma Fg levels were observed compared with the pretransfused plasma (time 0). Subsequently, the plasma Fg decreased gradually throughout the 4-day observation period (left panel). Platelet Fg was assayed before transfusion (0), as well as 2 and 4 days after transfusion. Significant levels of Fg were detected in Fg−/− platelets by day 2. Vinculin was used as a loading control (right panel). The immunoblots in each panel were developed from the same PVDF membrane and the experiments were repeated at least 3 times. (B) P-selectin expression on the surface of Fg−/− platelets before transfusion (shaded), as well as 1, 2, and 4 days after transfusion (arrow indicated), was analyzed via flow cytometry after incubation with either thrombin (left panel) or TRAP (right panel). Plasma Fg transfusion completely reversed the defect in P-selectin expression on Fg−/− platelets. (C) P-selectin expression on thrombin- or TRAP-activated platelets was plotted to show the time course. A significant increase in cell surface P-selectin expression on Fg−/− platelets was observed 1, 2, and 4 days after transfusion (n = 6, **P < .001), and demonstrated a tendency to increase with time following transfusion. Dashed line indicates cell surface P-selectin expression on wild-type platelets.
Fibrinogen transfusion recovered platelet surface P-selectin in Fg−/− mice. (A) Plasma Fg was determined by Western blot 30 minutes (30′), 6 hours (6h), 1 day (1d), 2 days (2d), and 4 days (4d) after transfusion. Thirty minutes after transfusion, significant plasma Fg levels were observed compared with the pretransfused plasma (time 0). Subsequently, the plasma Fg decreased gradually throughout the 4-day observation period (left panel). Platelet Fg was assayed before transfusion (0), as well as 2 and 4 days after transfusion. Significant levels of Fg were detected in Fg−/− platelets by day 2. Vinculin was used as a loading control (right panel). The immunoblots in each panel were developed from the same PVDF membrane and the experiments were repeated at least 3 times. (B) P-selectin expression on the surface of Fg−/− platelets before transfusion (shaded), as well as 1, 2, and 4 days after transfusion (arrow indicated), was analyzed via flow cytometry after incubation with either thrombin (left panel) or TRAP (right panel). Plasma Fg transfusion completely reversed the defect in P-selectin expression on Fg−/− platelets. (C) P-selectin expression on thrombin- or TRAP-activated platelets was plotted to show the time course. A significant increase in cell surface P-selectin expression on Fg−/− platelets was observed 1, 2, and 4 days after transfusion (n = 6, **P < .001), and demonstrated a tendency to increase with time following transfusion. Dashed line indicates cell surface P-selectin expression on wild-type platelets.
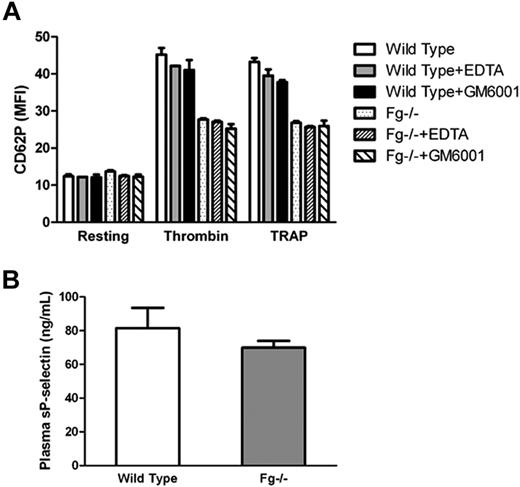
Impaired platelet surface P-selectin expression is unlikely due to excessive shedding
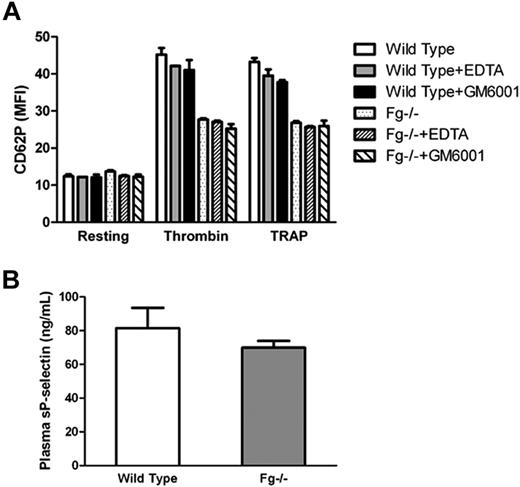
It has been reported that following translocation to the cell surface, P-selectin is shed via proteolytic cleavage resulting in an increase in soluble P-selectin. Therefore, we determined whether the observed surface P-selectin deficiency in vitro was due to increased shedding of P-selectin after activation of Fg−/− platelets. We incubated platelets with EDTA (10 mM; a divalent cation chelator), or the pan-specific matrix metalloproteinase inhibitor GM6001 (10 μM), and examined platelet P-selectin expression via flow cytometry. No significant difference in platelet surface P-selectin expression was observed on Fg−/− platelets in the presence or absence of EDTA or GM6001 (Figure 4A). There was no significant difference in soluble P-selectin in the supernatant of activated Fg−/− platelets compared with wild-type platelets (data not shown). Plasma-soluble P-selectin levels were also comparable between Fg−/− and wild-type mice (P = .26; Figure 4B). Our data suggest that proteolytic cleavage is not likely responsible for the impaired P-selectin expression observed on Fg−/− platelets.
Impaired platelet surface P-selectin expression is unlikely due excessive shedding. (A) Wild-type and Fg−/− platelets were incubated with or without either EDTA or GM6001 before addition of thrombin or TRAP. P-selectin expression on the platelet surface was analyzed via flow cytometry. The mean MFI ± SEM from the results was plotted. No significant difference in platelet surface P-selectin expression was observed on Fg−/− platelets in the presence or absence of EDTA or GM6001. (B) Soluble P-selectin in blood plasma was detected by ELISA. There was no significant difference between Fg−/− and wild-type mice (n = 3; P = .26).
Impaired platelet surface P-selectin expression is unlikely due excessive shedding. (A) Wild-type and Fg−/− platelets were incubated with or without either EDTA or GM6001 before addition of thrombin or TRAP. P-selectin expression on the platelet surface was analyzed via flow cytometry. The mean MFI ± SEM from the results was plotted. No significant difference in platelet surface P-selectin expression was observed on Fg−/− platelets in the presence or absence of EDTA or GM6001. (B) Soluble P-selectin in blood plasma was detected by ELISA. There was no significant difference between Fg−/− and wild-type mice (n = 3; P = .26).
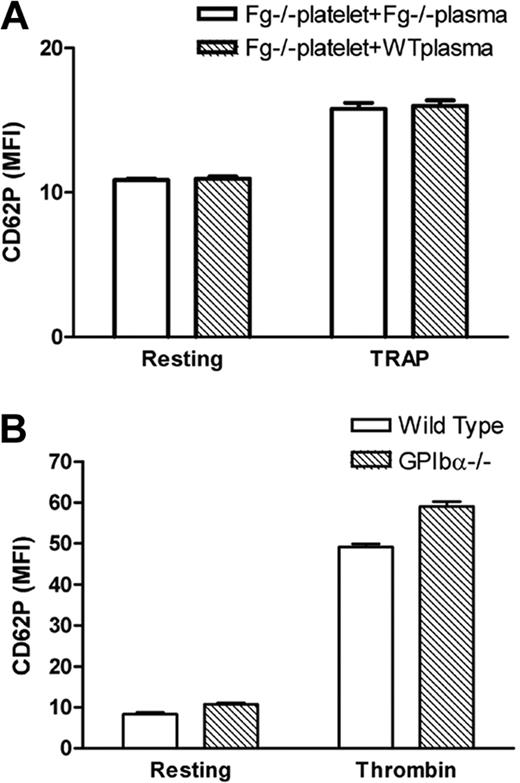
Impaired platelet surface P-selectin expression is unlikely due to deficient cell surface P-selectin binding, or defective platelet granule release
It is notable that P-selectin binds to sulfatides, and sulfatides in some Fg molecules26 may bind to P-selectin on the platelet surface after Fg is anchored to β3 integrin. To test this hypothesis, we incubated exogenous Fg or wild-type plasma with Fg−/− platelets for 60 minutes before TRAP treatment during flow cytometry assays. This short period of incubation with Fg was unable to reverse the deficiency of P-selectin expression on Fg−/− platelets (Figure 5A). This suggests that platelet β3 integrin-bound Fg does not play a role in retaining P-selectin on the cell surface, and therefore lack of Fg on the platelet surface does not explain the deficiency of P-selectin expression on Fg−/− platelets. These data are consistent with the results from EDTA-treated platelets (Figure 4A) in which the β3 integrin-Fg interaction on the platelet surface is diminished. Since there is no fibrin formation in TRAP-treated platelets,22 the deficiency of P-selectin expression (as shown in Figure 4A) is also unlikely due to fibrin-P-selectin interaction on the platelet surface. We also did not find a decrease in P-selectin expression on GPIbα−/− platelets (Figure 5B), which excludes possible indirect contributions of GPIbα-P-selectin or GPIbα-fibrin-P-selectin to the impaired P-selectin expression on Fg−/− platelets.
Impaired platelet surface P-selectin expression is unlikely due to deficient cell surface P-selectin binding. (A) Fg−/− platelets were incubated with either Fg−/− or wild-type plasma 60 minutes before addition of TRAP. There was no significant difference in Fg−/− platelet surface P-selectin expression in Fg−/− plasma or wild-type plasma (n = 3; P > .05). (B) P-selectin expression on gel-filtered wild-type and GPIbα−/− platelets was examined via flow cytometry. Higher expression of P-selectin on GPIbα−/− platelets likely reflects the larger size of GPIbα−/− platelets.
Impaired platelet surface P-selectin expression is unlikely due to deficient cell surface P-selectin binding. (A) Fg−/− platelets were incubated with either Fg−/− or wild-type plasma 60 minutes before addition of TRAP. There was no significant difference in Fg−/− platelet surface P-selectin expression in Fg−/− plasma or wild-type plasma (n = 3; P > .05). (B) P-selectin expression on gel-filtered wild-type and GPIbα−/− platelets was examined via flow cytometry. Higher expression of P-selectin on GPIbα−/− platelets likely reflects the larger size of GPIbα−/− platelets.
Although we have not studied the release of α-granule proteins due to the limitations of antimouse antibodies, no significant difference in ATP release was observed between Fg−/− platelets and littermate control platelets (data not shown). This suggests that platelet granule release is likely not impaired in Fg−/− platelets.
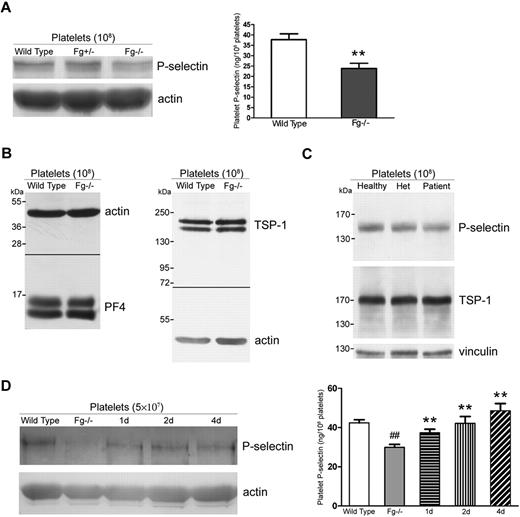
Impaired P-selectin expression on Fg−/− platelets reflects decreased platelet P-selectin content
To investigate whether the decreased P-selectin expression on Fg−/− platelets resulted from down-regulation of total platelet P-selectin, we examined P-selectin levels in wild-type (Fg+/+), Fg+/−, and Fg−/− platelets via Western blot and ELISA. Significantly less P-selectin was observed in Fg−/− platelets compared with Fg+/+ platelets (Figure 6A, P < .001). The decreased P-selectin content in Fg−/− mouse platelets seems to be specific since no decrease in other α-granule proteins such as PF4, TSP-1 (Figure 6B), and vitronectin was observed.6 Human subjects with an inherited Fg deficiency also revealed a decreased amount of total platelet P-selectin, whereas the amount of TSP-1 (Figure 6C) and vitronectin in platelets was normal.22
Impaired P-selectin expression on Fg−/− platelets reflects decreased platelet P-selectin content. (A) P-selectin levels in Fg+/+, Fg+/−, and Fg−/− mouse platelets were examined via Western blot. Actin was used as a loading control. A decrease in P-selectin was observed in Fg−/− compared with wild-type and Fg+/− platelets (left panel). Total platelet P-selectin levels were significantly decreased in Fg−/− platelets compared with wild-type platelets, as measured with ELISA (right panel; n = 3; **P < .001). (B) Total platelet PF4 and TSP-1 levels in Fg+/+ and Fg−/− mouse platelets were examined via Western blot. Actin was used as a loading control. No significant difference was observed. (C) Total platelet P-selectin levels in platelets of a healthy donor, the homozygous patient, and his mother (Het) were examined via Western blot. Vinculin was used as a loading control. A decrease in P-selectin was observed in the patient's platelets. A slight decrease was also observed in platelets of the heterozygous parent. No significant difference in the levels of TSP-1 was observed. (D) Total platelet P-selectin levels were examined via Western blot (left panel) and ELISA (right panel). Plasma Fg transfusion restored total P-selectin levels in Fg−/− platelets 1, 2, and 4 days after transfusion. A significant decrease in Fg−/− compared with wild-type platelets was observed via ELISA (n = 3; ##P < .001). Transfusion of plasma Fg significantly increased total platelet P-selectin levels 1, 2, and 4 days after transfusion compared with untreated Fg−/− platelets (shaded) (n = 3; **P < .001). The immunoblots in each panel were developed from the same PVDF (A, C-D) or nitrocellulose (B) membrane and the experiments were repeated at least 3 times.
Impaired P-selectin expression on Fg−/− platelets reflects decreased platelet P-selectin content. (A) P-selectin levels in Fg+/+, Fg+/−, and Fg−/− mouse platelets were examined via Western blot. Actin was used as a loading control. A decrease in P-selectin was observed in Fg−/− compared with wild-type and Fg+/− platelets (left panel). Total platelet P-selectin levels were significantly decreased in Fg−/− platelets compared with wild-type platelets, as measured with ELISA (right panel; n = 3; **P < .001). (B) Total platelet PF4 and TSP-1 levels in Fg+/+ and Fg−/− mouse platelets were examined via Western blot. Actin was used as a loading control. No significant difference was observed. (C) Total platelet P-selectin levels in platelets of a healthy donor, the homozygous patient, and his mother (Het) were examined via Western blot. Vinculin was used as a loading control. A decrease in P-selectin was observed in the patient's platelets. A slight decrease was also observed in platelets of the heterozygous parent. No significant difference in the levels of TSP-1 was observed. (D) Total platelet P-selectin levels were examined via Western blot (left panel) and ELISA (right panel). Plasma Fg transfusion restored total P-selectin levels in Fg−/− platelets 1, 2, and 4 days after transfusion. A significant decrease in Fg−/− compared with wild-type platelets was observed via ELISA (n = 3; ##P < .001). Transfusion of plasma Fg significantly increased total platelet P-selectin levels 1, 2, and 4 days after transfusion compared with untreated Fg−/− platelets (shaded) (n = 3; **P < .001). The immunoblots in each panel were developed from the same PVDF (A, C-D) or nitrocellulose (B) membrane and the experiments were repeated at least 3 times.
We further examined platelet P-selectin content in Fg−/− mice after Fg transfusion and found that Fg indeed restored the total intracellular P-selectin level. Increased platelet P-selectin was observed 1 day after transfusion, and the level of P-selectin continued to increase 2 and 4 days after transfusion (Figure 6D). Platelet P-selectin content reached levels similar to wild-type 2 days after transfusion (Figure 6D right panel).
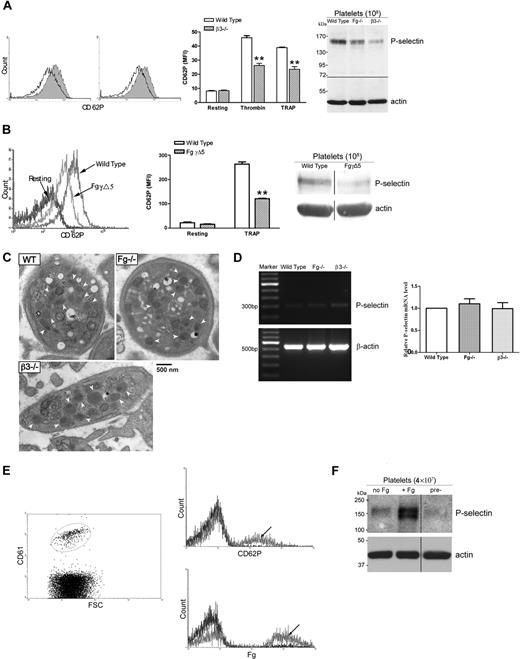
Engagement of β3 integrin and Fg is required for maintenance of platelet intracellular and cell surface P-selectin expression
As shown in Figure 5A, β3 integrin–bound Fg or fibrin is likely not the binding site required for P-selectin cell surface retention. However, we demonstrated significantly lower P-selectin expression on β3−/− platelets after activation compared with wild-type controls (Figure 7A), which is consistent with earlier studies using β3−/− mice of a different background.27 The decreased platelet P-selectin content was also detected by both Western blot (Figure 7A right panel) and ELISA (data not shown). There was no increase in P-selectin expression on platelets from β3−/− mice after Fg transfusion (data not shown). These data suggest that β3 integrin is involved in intracellular P-selectin expression and that long-term interaction between Fg and platelet β3 integrin may be required for this process.
Engagement of β3 integrin and Fg is required for maintenance of platelet cell surface and intracellular P-selectin expression. (A) Gel-filtered platelets were incubated with either thrombin (left histogram) or TRAP (right histogram), and P-selectin expression on the platelet surface was analyzed via flow cytometry. P-selectin expression was significantly decreased on β3−/− platelets (black) after activation compared with wild type (shaded); bar graph indicates MFI plus or minus SEM (n = 3; **P < .01). A decrease in total platelet P-selectin levels in β3−/− platelets was also observed by immunoblot (right panel). The immunoblots in each panel were developed from the same nitrocellulose membrane and the experiments were repeated at least 3 times. (B) P-selectin expression was also significantly decreased on FgγΔ5 platelets after TRAP activation compared with wild type (left panel, arrow indicated); bar graph indicates MFI plus or minus SEM (middle panel) (n = 4; **P < .001). A decrease in total platelet P-selectin levels in FgγΔ5 platelets was also observed by immunoblot (right panel). The immunoblots were developed from the same PVDF membrane and the experiments were repeated at least 3 times. (C) No significant differences in the average number of platelet α-granules (arrow indicated) in Fg−/− and β3−/− mice compared with those of wild-type (WT) mice were observed (P > .05). The quantitative morphometric comparisons were performed via electron microscopy in 50 platelets per mouse and 2 mice in each group were quantitated. (D) Total platelet mRNA was isolated and reverse transcribed. PCR amplification of cDNA was performed using primers for mouse P-selectin and β-actin (internal control). The levels of P-selectin mRNA in Fg−/− and β3−/− platelets appear similar to wild-type controls after individual bands are normalized to their β-actin controls (left panel). These results were confirmed by quantitative real-time PCR (Fg−/− versus WT: P = .44; β3−/− versus WT: P = .96; right panel). (E) Gel-filtered platelets from Fg−/− mice (β3 integrin positive) were transfused to β3−/− mice (Fg positive) and Fg−/− mice (negative control). Two days after transfusion, platelets were isolated and analyzed via flow cytometry. β3 integrin–positive Fg−/− platelets were gated (left panel), and a significant increase in Fg and P-selectin expression was observed on those β3 integrin–positive platelets isolated from β3−/− mice (arrow indicated), but not from Fg−/− mice, after treatment with TRAP (right panel). The experiments were repeated at least 3 times. (F) Washed platelets from Fg−/− mice were incubated in culture media supplemented with Fg (+ Fg) or media alone (no Fg). Platelet P-selectin levels in cultured platelets and uncultured platelets (pre-: platelets were lysed prior to incubation) were examined via Western blot. Platelets cultured with Fg had significantly greater levels of P-selectin. The immunoblots were developed from the same PVDF membrane and the experiments were repeated at least 3 times.
Engagement of β3 integrin and Fg is required for maintenance of platelet cell surface and intracellular P-selectin expression. (A) Gel-filtered platelets were incubated with either thrombin (left histogram) or TRAP (right histogram), and P-selectin expression on the platelet surface was analyzed via flow cytometry. P-selectin expression was significantly decreased on β3−/− platelets (black) after activation compared with wild type (shaded); bar graph indicates MFI plus or minus SEM (n = 3; **P < .01). A decrease in total platelet P-selectin levels in β3−/− platelets was also observed by immunoblot (right panel). The immunoblots in each panel were developed from the same nitrocellulose membrane and the experiments were repeated at least 3 times. (B) P-selectin expression was also significantly decreased on FgγΔ5 platelets after TRAP activation compared with wild type (left panel, arrow indicated); bar graph indicates MFI plus or minus SEM (middle panel) (n = 4; **P < .001). A decrease in total platelet P-selectin levels in FgγΔ5 platelets was also observed by immunoblot (right panel). The immunoblots were developed from the same PVDF membrane and the experiments were repeated at least 3 times. (C) No significant differences in the average number of platelet α-granules (arrow indicated) in Fg−/− and β3−/− mice compared with those of wild-type (WT) mice were observed (P > .05). The quantitative morphometric comparisons were performed via electron microscopy in 50 platelets per mouse and 2 mice in each group were quantitated. (D) Total platelet mRNA was isolated and reverse transcribed. PCR amplification of cDNA was performed using primers for mouse P-selectin and β-actin (internal control). The levels of P-selectin mRNA in Fg−/− and β3−/− platelets appear similar to wild-type controls after individual bands are normalized to their β-actin controls (left panel). These results were confirmed by quantitative real-time PCR (Fg−/− versus WT: P = .44; β3−/− versus WT: P = .96; right panel). (E) Gel-filtered platelets from Fg−/− mice (β3 integrin positive) were transfused to β3−/− mice (Fg positive) and Fg−/− mice (negative control). Two days after transfusion, platelets were isolated and analyzed via flow cytometry. β3 integrin–positive Fg−/− platelets were gated (left panel), and a significant increase in Fg and P-selectin expression was observed on those β3 integrin–positive platelets isolated from β3−/− mice (arrow indicated), but not from Fg−/− mice, after treatment with TRAP (right panel). The experiments were repeated at least 3 times. (F) Washed platelets from Fg−/− mice were incubated in culture media supplemented with Fg (+ Fg) or media alone (no Fg). Platelet P-selectin levels in cultured platelets and uncultured platelets (pre-: platelets were lysed prior to incubation) were examined via Western blot. Platelets cultured with Fg had significantly greater levels of P-selectin. The immunoblots were developed from the same PVDF membrane and the experiments were repeated at least 3 times.
It has been demonstrated that the C-terminus of the Fg γ chain contains a critical binding site for platelet β3 integrin. Deletion of the last 5 residues (QAGDV) in this region (FgγΔ5) abolished platelet aggregation in anticoagulated blood19 and blocked Fg internalization.20 This deletion does not affect fibrin clotting and clot retraction, suggesting that other β3 integrin binding sites still exist in FgγΔ5, which support platelet β3 integrin–mediated clot retraction. To map the critical residues on Fg that may control P-selectin expression, platelets in PRP of FgγΔ5 mice were stimulated with TRAP (0.5-2 mM) and P-selectin expression was measured via flow cytometry. As shown in Figure 7B, P-selectin expression on FgγΔ5 platelets was significantly lower than that of wild-type control platelets. Similar to the platelets in Fg−/− and β3−/− mice, the platelet P-selectin content in FgγΔ5 mice was also decreased (Figure 7B right panel). Thus, the C-terminus of the Fg γ chain but not other binding sites, including the multiple RGD sites, appears to be required for maintaining the levels of intracellular P-selectin in platelets.
To examine whether the decreased platelet P-selectin expression was due to the impairment of biogenesis of platelet α-granules, we quantitated α-granules in Fg−/− and β3−/− platelets via electron microscopy. The total α-granule content in platelets from wild-type, Fg−/−, and β3−/− mice is similar (mean ± SD: wild type, 7.3 ± 4.4; Fg−/−, 6.0 ± 5.4; β3−/−, 6.9 ± 3.6) as shown in representative electron microscopy images (Figure 7C). These results reveal that there is no significant difference (P > .05) in the average number of platelet α-granules in these mice.
We then examined whether P-selectin mRNA is present within platelets, and if so, whether variations in the levels of this mRNA could explain the decreased platelet P-selectin content. As shown in Figure 7D (left panel), P-selectin cDNA was selectively amplified and the levels of P-selectin mRNA in Fg−/− and β3−/− platelets were similar to wild-type controls after bands were normalized to β-actin internal controls. These results were further confirmed by quantitative real-time PCR using different primers for mouse P-selectin and β-actin (Figure 7D right panel). This suggests that megakaryocytic transcription of the gene for P-selectin or packaging of P-selectin mRNA into newly formed platelets is likely not impaired in the absence of Fg.
To further investigate the mechanism(s) involved in the restoration of platelet P-selectin after addition of Fg, gel-filtered platelets from Fg−/− mice (β3 integrin positive) were transfused to β3−/− mice (Fg positive) and Fg−/− mice (negative control). After 2-day circulation in β3−/− mice, but not in Fg−/− mice, the β3 integrin–positive platelets (Figure 7E left panel) demonstrated significantly increased Fg and P-selectin expression after thrombin (data not shown) or TRAP (Figure 7E right panel) treatment. The multiple peaks in Figure 7E may reflect different populations of platelets, which have variable potential for P-selectin synthesis. This demonstrates that Fg−/− platelets themselves (ie, not necessarily via megakaryocytes) are able to internalize Fg and up-regulate P-selectin expression in the presence of Fg.
To confirm the ability of platelets to up-regulate P-selectin, Fg−/− platelets were incubated in cell culture media overnight and platelet P-selectin was detected via Western blot. Our data showed that following incubation with media containing Fg, the levels of platelet P-selectin were greater than that of both platelets incubated in media alone and platelets lysed prior to incubation (Figure 7F). An increase in P-selectin was also observed when wild-type platelets were cultured under similar conditions (data not shown). These data demonstrated that P-selectin synthesis occurs in platelets following interaction with Fg.
Discussion
Platelet P-selectin is involved in multiple physiological processes including platelet aggregation, platelet-leukocyte and platelet-endothelial cell interactions, and others4,8,28,29 Clinically, P-selectin is widely accepted as a marker of platelet activation, and elevated levels of plasma P-selectin in thrombotic disorders result mainly from P-selectin shedding from the platelet surface.30,31 However, there is as yet little insight regarding the mechanisms that regulate platelet P-selectin expression. In this study, we found that Fg promotes P-selectin expression on both human and murine platelets after platelet activation. We demonstrated that the observed deficiency of P-selectin expression was likely not due to increased proteolytic shedding, deficiency of cell surface Fg-mediated P-selectin binding, or impairment of platelet granule release, but rather due to decreased platelet P-selectin content. Transfusion of plasma Fg completely reversed the defect in platelet P-selectin expression. We found that interaction of platelet β3 integrin and the C-terminus of the Fg γ chain is required to maintain the levels of platelet intracellular and cell surface P-selectin expression. Most importantly, we demonstrate that platelets, not necessarily via megakaryocytes, are able to internalize Fg and up-regulate P-selectin expression.
Fg is the key molecule in hemostasis and thrombosis. Fg also plays roles in pathophysiological processes, such as infection,32 wound healing,33 and development of atherosclerotic plaques.34 Recently, it was shown that inflammatory responses to endotoxin were delayed in Fg-deficient mice, which suggests a role of Fg as a mediator of cross-talk between coagulation and inflammation.35 To explain the role of Fg in inflammation, most studies focus on fibrin deposition, stimulation of chemokine secretion, and the facilitation of neutrophil-endothelial interaction in septic/endotoxemic shock.36,37 A recent study demonstrated that infusion of activated platelets caused Weibel-Palade body release leading to P-selectin–mediated leukocyte rolling,28 suggesting that platelet P-selectin is crucial in the process of inflammation. Our study clearly demonstrated that Fg enhances platelet intracellular P-selectin levels and affects P-selectin expression on the surface of mouse and human platelets, which may partially explain the role of Fg in inflammation, hemostasis, and atherosclerosis.
The role of platelets in the immune response has recently been highlighted. Platelets not only facilitate lymphocyte homing,1 but also affect B-lymphocyte differentiation and antibody class switch via the high platelet CD40L content.2 Although the physiological role of large amounts of TGF-β (potent immunosuppressive factor) in platelets is currently unclear, platelets may also contribute to immune regulation via different granule sorting/release pathways.38,39 Since P-selectin is involved in all of these platelet-leukocyte interactions, and P-selectin promotes the Th-1–like immune response,40 our finding in this study may be of general interest in the field of immune-related inflammation.
There are some differences between the observed phenomenon in the mouse model and the human subjects. P-selectin expression on heterozygous (Fg+/−) mouse platelets was comparable with wild-type (Fg+/+) controls, whereas that on platelets of the heterozygous hypofibrinogenemic patients was significantly less than the healthy controls. An explanation for this discrepancy may lie in the different gene mutations affecting plasma Fg levels in these 2 species. The Fg−/− mice were generated via deletion of the Fg α chain and, as a result, 75% of plasma Fg remains in heterozygous mice, whereas the severely hypofibrinogenemic patient has a mutation in the Fg β chain,21 which controls Fg secretion. Therefore, only 50% of normal levels of plasma Fg remain in the heterozygous hypofibrinogenemic parents. Different amounts of plasma Fg may account for the difference in observed P-selectin levels, although we cannot exclude the possibility of other effects attributed to the Fg β chain. We also cannot exclude the possibility that human platelet surface P-selectin expression may be more sensitive to variations in plasma levels of Fg since the volume of human platelets is 2-fold larger than mouse, but the platelet counts are 4-fold lower. The distribution of platelet P-selectin may be different between these 2 species. It is therefore worthwhile to further study the relationship between plasma Fg levels and platelet P-selectin expression in different human populations, including afibrinogenemic and hypofibrinogenemic patients, before and after Fg transfusion. In addition, the concentration of Fg in the blood varies 2- to 4-fold in healthy human populations, and can be significantly elevated after inflammation, including multiple common diseases such as stroke and diabetes.41 These variations may significantly affect platelet intracellular and cell surface P-selectin expression. Our data suggest that P-selectin as an activation marker on platelets should be used with caution. This may be particularly important with respect to patients with hypofibrinogenemia, afibrinogenemia, Glanzmann thrombasthenia (αIIbβ3 integrin deficiency), hyperfibrinogenemia, or inflammation.
Patients with type I Glanzmann thrombasthenia completely lack platelet α-granule Fg,42 confirming the notion that Fg is internalized by megakaryocytes and platelets via αIIbβ3 integrin. Our previous study further demonstrated that engagement of the C-terminus of the Fg γ chain and β3 integrin is required for Fg internalization, and little Fg is detectable in β3−/− and FgγΔ5 platelets.20 It is curious that although patients with Glanzmann thrombasthenia often have a serious bleeding phenotype, there is no report regarding whether they suffer from increased infections.42,43 However, it has been reported that β3 integrin may play an important role in supporting the Th-1 immune response and induction of IL-12 can be inhibited by both peptide GRGDS and antibodies against the integrin β3 subunit.44 It is currently unclear whether impairment of platelet P-selectin expression follows after treatment with these β3 integrin antagonists and whether this contributes to the alteration of the immune response.
P-selectin is trafficked from the Golgi complex to α-granules in cultured human megakaryocytes.45 That platelet α-granules are required for the presence of P-selectin in platelets is noted from α-granule–deficient ARC (arthrogryposis, renal dysfunction, and cholestasis) syndrome patients whose platelets completely lack P-selectin.23 Although nearly all platelet α-granules contain P-selectin, Fg is present in only a fraction of platelet α-granules.38 Our data did not detect significant differences in the average number of platelet α-granules in Fg−/− and β3−/− mouse platelets compared with those of wild-type mice (Figure 7C), which is consistent with the immunoblot data suggesting that no other α-granule proteins were decreased (Figure 6B-C). However, albeit statistically insignificant, it is notable that there were slightly decreased mean numbers of platelet α-granules in Fg−/− and β3−/− mice. Considering the technical limitations of accurately quantitating platelet α-granules using electron microscopy, we cannot completely exclude that altered α-granule biogenesis could be one possible mechanism of regulating P-selectin expression in platelets.
The significant decrease in total platelet P-selectin in Fg−/− mice (Figure 6A) and the ability of Fg transfusion to completely restore the observed defect (Figure 6D) are intriguing. Since a similar defect in P-selectin expression was also observed in β3−/− and FgγΔ5 mice (Figure 7A-B), this suggests that interaction between platelet β3 integrin and the C-terminus of the Fg γ chain plays an important role in regulating intracellular platelet P-selectin levels. We propose that the interaction between Fg and β3 integrin provides a signal for de novo synthesis of P-selectin; however, we cannot exclude that internalized Fg may also stabilize granule P-selectin in the secretory pool and protect it from degradation. Although we are not able to exclude the latter possibility in the present study, the maintenance of platelet P-selectin by Fg should occur primarily in platelets and not megakaryocytes. This is because the levels of intracellular P-selectin increased vigorously 1 day after Fg transfusion and reached more than 80% of the normal level by 2 days after transfusion. This is far shorter than the half-life of murine platelets and cannot be well explained by either enhancement of platelet production (eg, with higher intracellular P-selectin) or aged platelet destruction. Our data (Figure 7D-F) clearly demonstrate that platelets contain P-selectin mRNA and are able to increase P-selectin expression, likely via de novo synthesis, after they enter Fg-positive environments (eg, β3−/− mice or Fg-supplemented media). These data are consistent with earlier studies that platelets are able to translate constitutive mRNAs into proteins, including cytokines and β3 integrin,17,18,46,47 and that outside-in signals from β3 integrin play an important role in this process.47 It is notable that platelets cultured overnight in the absence of Fg seemed to increase P-selectin content, although to a much lesser extent than those incubated with Fg (Figure 7F), suggesting that resting platelets are able to synthesize this protein in vitro. Thus, enhancing de novo synthesis of P-selectin in platelets is likely the major mechanism by which Fg up-regulates platelet P-selectin expression, although the same mechanism may also occur in megakaryocytes and may promote α-granule biogenesis.
Although there is only a 40% to 60% decrease in the level of P-selectin on Fg−/− platelets, this defect seems to be more remarkable on human platelets. It is currently unknown whether the decreased platelet P-selectin expression contributes to embolization observed in afibrinogenemic patients,48 as well as the embolization and platelet aggregate dissociation in Fg−/− mice observed in our intravital microscopy thrombosis model.6,20 It is also unclear whether the 40% to 60% alteration in platelet P-selectin is sufficient to affect long-term pathological processes, such as atherosclerosis, diabetes, and others. These important questions deserve further investigation.
In summary, we demonstrated that P-selectin expression was significantly decreased in Fg-deficient human and mouse platelets. We found that β3 integrin engagement with the C-terminus of the Fg γ chain, but not its RGD sequences or other integrin binding sites, is required to maintain normal levels of platelet intracellular P-selectin, likely via de novo P-selectin synthesis. Whether this Fg-β3 integrin interaction also affects other platelet proteins beyond the ones tested in our studies, and whether the altered intracellular P-selectin level plays significant roles in thrombosis, hemostasis, and inflammation, including immune-mediated inflammation, requires further study.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank D. D. Wagner, C. V. Denis, R. O. Hynes, J. Ware, and Z. M. Ruggeri for their earlier work preparing gene-deficient (VWF−/−, β3−/−, and GPIbα−/−) mice, and M.L. Webster for assistance with preparation of the paper. We also thank X. C. Xu for his assistance in human platelet sample preparation.
This work was supported in part by the Heart and Stroke Foundation of Canada (Ottawa, ON); Canadian Institutes of Health Research (CIHR) and Canadian Blood Services; Equipment Funds from St Michael's Hospital, Canadian Blood Services, and Canada Foundation for Innovation; and International Cooperation Research Fund of Anhui Provincial Scientific and Technologic Committee (China). W.H.A.K. was supported by an operating grant from CIHR and a Phase II Clinician Scientist Salary Award from the Heart and Stroke Foundation of Ontario. H.Y. is a recipient of the Heart and Stroke/Richard Lewar Excellence award and a Canadian Blood Services postdoctoral fellowship award. S.L. is a recipient of the Canadian Blood Services summer internship program and the Master's Studentship Award of the Heart and Stroke Foundation of Ontario.
Authorship
Contribution: H.Y. designed the experiments, performed the research, analyzed the data, and wrote the paper; S.L. performed the research and assisted in paper preparation; Z.Z. performed the research on human subjects; L.L. performed some of the research in the revised paper; W.H.A.K. analyzed data and edited the paper; P.C. and J.B. performed the research; C.M.S. performed the research and edited the paper; M.J.F. and J.L.D. performed the research and analyzed the data with FgγΔ5 mice; J.F. contributed a vital analytical tool (flow cytometer), analyzed the data, and edited the paper; and H.N. is the principal investigator who designed the experiments, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Heyu Ni, Canadian Blood Services and Department of Laboratory Medicine and Pathobiology, St Michael's Hospital, University of Toronto, 30 Bond St, Rm 2-006, Bond Wing, Toronto, ON, Canada M5B 1W8; e-mail: nih@smh.toronto.on.ca.