Abstract
Background: The incorporation of intensive chemotherapy, hematopoietic stem cell transplantation (HSCT), targeted therapies including rituximab and tyrosine kinase inhibitors contributes substantial improvement in the outcome of patients with ALL over decades. VAD was changed to hyper-CVAD in 1992; rituximab was added to hyper-CVAD for CD20 positive ALL in 1999/2000; inotuzumab ozogamicin in combination with low-intensity chemotherapy was offered to elderly patients starting in 2011. The aim of this study is to describe the outcome of patients with ALL over decades by age groups.
Methods: From 1980 to 2016, patients with newly diagnosed ALL at our institution were analyzed. Burkitt leukemia was excluded from our analysis. Patients were divided into age groups as follows: age 15-39, age 40-60, and age >60. Patients were subsequently divided into diagnostic year cohorts by decade: 1980-1989, 1990-1999, 2000-2009, and 2010-2016. Overall survival was defined as time interval from diagnosis to the date of death regardless of any cause. Kaplan-Meier method with a log-rank test was used for survival comparison between cohorts. Stepwise multivariate analysis with Cox proportional hazards model was used to evaluate the impact of diagnosis year on OS. P-values were two-sided, and a p-value less than 0.05 was considered statistically significant.
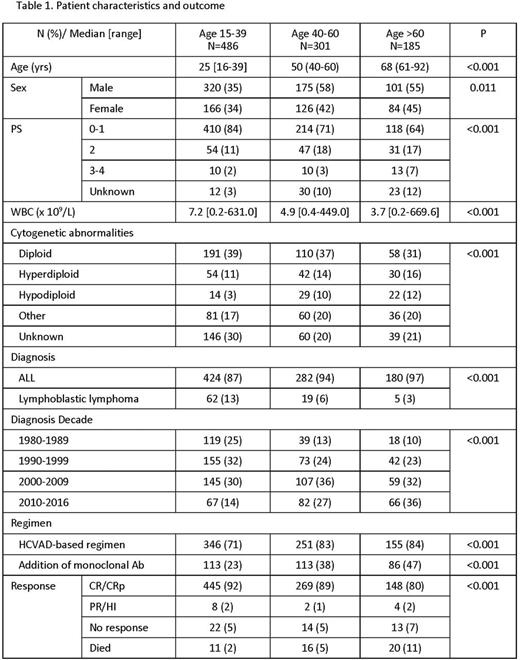
Results: Overall, 972 patients were identified and analyzed in our study. Median age at diagnosis was 39.5 years (range, 16-92) with a median follow-up of 10.4 years (range, 0.0-31.3). Patients were divided into 486 patients (50%) in the age 15-39 category, 301 patients (31%) in the age 40-60 category, and 185 patients (19%) in the age over 60 category. Baseline patient characteristics are summarized in Table 1. Overall, the median OS durations were 4.5 years, 2.8 years, and 1.3 years in age 15-39, age 40-60, and age >60, respectively (p<0.001; p<0.001). Of the 486 patients in age 15-39, the improvement in OS was observed from 1980-1989 to 1990-1999 (p<0.001); of the 301 patients in age 40-60, from 1980-1989 to 1990-1999, and from 1990-1999 to 2000-2009 (p=0.042; p=0.003); of the 185 patients in age >60, from 2000-2009 to 2010-2016 (p=0.039). Stepwise multivariate analysis identified leukocytosis, thrombocytopenia, hypoalbuminemia, elderly age, poor performance status, lack of complete response, and diagnostic year as adverse prognostic factors for OS. Each year since 1980 had an impact on OS with hazard ratio of 0.961 (95% confidence interval, 0.951-0.971; p<0.001).
Conclusion: Patients with ALL have significant improvement in OS throughout all ages over decades. However, the decade time points of improvement in OS were different between age cohorts. Different treatment strategy and clinical trial designs by each age group are needed for further improvement in patient's outcome.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Ravandi:BMS: Research Funding; Seattle Genetics: Consultancy, Honoraria, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Wierda:Novartis: Research Funding; Abbvie: Research Funding; Acerta: Research Funding; Gilead: Research Funding; Genentech: Research Funding. Konopleva:Cellectis: Research Funding; Calithera: Research Funding. Pemmaraju:incyte: Consultancy, Honoraria, Research Funding; novartis: Consultancy, Honoraria, Research Funding; LFB: Consultancy, Honoraria; stemline: Consultancy, Honoraria, Research Funding; cellectis: Consultancy, Research Funding; affymetrix: Research Funding. DiNardo:Agios: Other: advisory board, Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Celgene: Research Funding; Abbvie: Research Funding; Novartis: Other: advisory board, Research Funding. Jain:Genentech: Research Funding; Novartis: Consultancy, Honoraria; Pharmacyclics: Consultancy, Honoraria, Research Funding; Novimmune: Consultancy, Honoraria; Seattle Genetics: Research Funding; Abbvie: Research Funding; Servier: Consultancy, Honoraria; Celgene: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Infinity: Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Incyte: Research Funding. Andreeff:Oncoceutics: Equity Ownership, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.