Abstract

INTRODUCTION: Hypomethylating agents (HMA) such as azacitidine and decitabine remain the standard of care for the treatment of myelodysplastic syndromes (MDS). Although responses are seen in 40-60% patients, median response duration is 12-14 months. Eventual loss of response is associated with poor outcomes. Multiple studies have tried do identify biomarkers of response. Presence of TET2 mutations has been associated to increased response in several studies. Clear identification of predictors of response is still required.
METHODS: We evaluated a total of 180 previously untreated patients with MDS or CMML that received HMA therapy at The University of Texas MD Anderson Cancer Center. Next generation sequencing (NGS), analyzing a panel of 28 genes (ABL1, ASXL1, BRAF, DNMT3A, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, IKZF2, JAK2, KIT, KRAS, MDM2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TET2, TP53 and WT1) was performed prior to therapy with HMA. Clinical and demographic data was obtained from clinical records. Generalized linear models were used to study association of response rates (ORR=overall and CR=complete) and risk factors. Response was defined following 2006 IWG criteria. The Kaplan-Meier produce limit methods were used to estimate the median overall survival (OS) and leukemia-free survival (LFS).
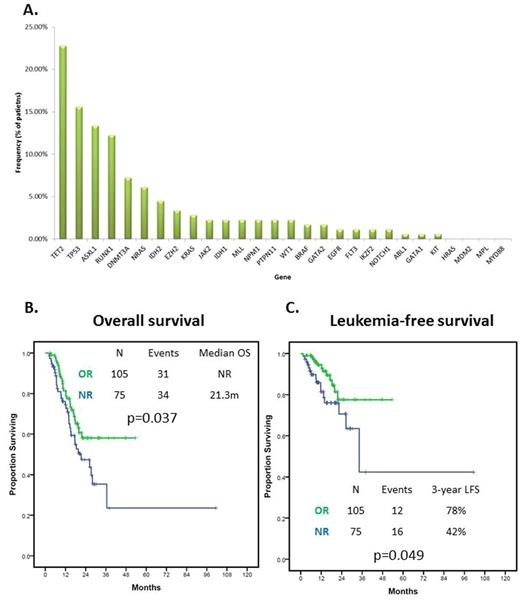
RESULTS: Patient characteristics are shown in Table 1. Median age at diagnosis was 67 years (range 20-88). A total of 108 patients (60%) had lower-risk MDS based on IPSS and 72 (40%) had higher-risk MDS. A total of 143 patients (79%) had MDS and 37 (21%) had CMML. Eighty-seven (49%) patients had normal karyotype with 36 (20%) having complex karyotype. Therapy consisted in azacitidine monotherapy in 60 (33%) patients, decitabine monotherapy in 55 (31%) and guadecitabine or combinations in 65 (36%). The ORR was 58% (105/180) with 66 (37%) patients achieving CR. A total of 123 (68%) patients had at least one detectable mutation. Median number of mutations was 1 (range 0-5). The most frequently detected mutations included TET2 (23%), TP53 (16%) and RUNX1 (12%) which were all present in >10% cases. Frequency of detected mutations is shown in Figure 1A. Presence of ASXL1 mutation was associated with decreased likelihood of achieving CR (OR 0.30, 95% CI 0.10-0.93, p=0.037). No differences in ORR were observed based on presence of any individual mutation or any other clinical characteristic. Presence of TET2 mutation was not significantly associated with an increased likelihood of response (OR 1.32, 95% CI 0.64-2.70, p=0.453) or CR (OR 1.30, 95% CI 0.64-2.65, p=0.469). We subsequently analyzed the impact of number of detected mutations on response to therapy. Presence of three or more detectable mutations was associated with lower ORR (OR 0.29, 95% CI 0.10-0.88, p=0.028) and a trend to lower likelihood of achieving a CR (OR 0.22, 95% CI 0.05-1.01, p=0.052). Mutations in ASXL1 (p=0.003), BRAF (p=0.021), DNMT3A (p=0.018), EZH2 (p=0.01), GATA2 (p=0.021), MLL (p<0.001), NRAS (p<0.001), PTPN11 (p=0.002), RUNX1 (p=0.015), TET2 (p=0.001) and WT1 (p=0.04) tended to appear in patients with three or more mutations. Presence of TET2 mutations did not predict for ORR (OR 1.83, 95% CI 0.78-4.25, p=0.163) or CR (OR 1.75, 95% CI 0.80-3.81, p=0.159) within cases with less than three mutations. The median follow up of the cohort was 14.5 months (range 2.4-101.3 months). Patients who did not achieve a response had significantly shorter OS (median OS NR vs 21.3 months, HR 1.68, 95% CI 1.03-2.73, p=0.037) (Figure 1B) and LFS (median LFS NR vs 34.3 months, HR 2.13, 95% CI 1.00-4.50, p=0.049) (Figure 1C). Achievement of a CR predicted for improved OS in patients with higher-risk MDS (median OS NR vs 14.6 months, p=0.046) but not in lower-risk patients (NR vs 27.3 months, p=0.239). No significant differences in LFS were observed based on achievement of CR in both higher (p=0.238) and lower risk patients (p=0.453).
CONCLUSION: The number of driver mutations may be a new biomarker to predict response to therapy with HMA in patients with MDS and CMML. Presence of ASXL1 mutations may be associated with a decreased risk of achieving a CR. As previously reported, response to therapy with HMA impacts the OS and LFS of patients with MDS. Incorporating sequencing data at diagnosis may help predict response to therapy and patient outcomes.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. DiNardo:Celgene: Research Funding; Daiichi Sankyo: Other: advisory board, Research Funding; Novartis: Other: advisory board, Research Funding; Abbvie: Research Funding; Agios: Other: advisory board, Research Funding. Daver:Sunesis: Consultancy, Research Funding; Ariad: Research Funding; Kiromic: Research Funding; Pfizer: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; BMS: Research Funding; Karyopharm: Honoraria, Research Funding. Konopleva:Cellectis: Research Funding; Calithera: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract