Abstract
GATA-1 germline mutation in mice results in embryonic lethality due to defective erythroid cell maturation, and thus other hematopoietic GATA factors do not compensate for the loss of GATA-1. To determine whether the obligate presence of GATA-1 in erythroid cells is due to its distinct biochemical properties or spatiotemporal patterning, we attempted to rescue GATA-1 mutant mice with hematopoietic GATA factor complementary DNAs (cDNAs) placed under the transcriptional control of the GATA-1gene. We found that transgenic expression of a GATA-1 cDNA fully abrogated the GATA-1–deficient phenotype. Surprisingly, GATA-2 and GATA-3 factors expressed from the same regulatory cassette also rescued the embryonic lethal phenotype of the GATA-1 mutation. However, adult mice rescued with the latter transgenes developed anemia, while GATA-1 transgenic mice did not. These results demonstrate that the transcriptional control dictating proper GATA-1 accumulation is the most critical determinant of GATA-1 activity during erythropoiesis. The results also show that there are biochemical distinctions among the hematopoietic GATA proteins and that during adult hematopoiesis the hematopoietic GATA factors are not functionally equivalent.
Of the 6 GATA factors, GATA-1, GATA-2, and GATA-3 constitute a distinct subfamily because they are expressed in hematopoietic lineages and because they share a similar gene organization. However, the amino acid sequence of GATA-1 has diverged significantly from that of the GATA-2 and GATA-3 proteins, which are more similar to one another.1 Within the hematopoietic compartment, GATA-1 is expressed in erythroid, megakaryocytic, eosinophilic, and mast cells2-5 in addition to Sertoli cells of the testis.6 The GATA-1 gene is transcribed from the distal testis first exon (IT) and the proximal erythroid first exon (IE) in Sertoli cells or hematopoietic cells, respectively.6 7
Employing a promoter interference approach, we recently generated an erythroid promoter-specific mutant allele of the GATA-1 gene (which we called GATA-1.05 because it was expressed at approximately 5% of wild-type [WT] level8). BecauseGATA-1 is located on the X chromosome,9 all male embryos hemizygous for the mutation died by 12.5 embryonic days (E12.5) due to arrest of primitive erythropoiesis, as observed in GATA-1–null mutant embryos.10 Subsequent analyses of GATA-1.05heterozygous female mice11 or lineage-specificGATA-1 mutant mice12 showed that GATA-1 was also vital for terminal megakaryocytic differentiation.
Although the expression of GATA-2 substantially overlaps that of GATA-1 in hematopoietic lineages,2,13GATA-1 gene disruption experiments clearly demonstrated that GATA-2 does not compensate for the loss of GATA-1 function in vivo.8,10Interestingly, in vitro differentiation of GATA-1–deficient embryonic stem (ES) cells revealed a 50-fold induction of GATA-2 messenger RNA (mRNA) in erythroid cells.14 15
The functional significance attributed to differences in the biochemical activities of GATA family members has been addressed in cell culture experiments. The first indication that these proteins might have distinct characteristics came from a study in which the GATA-1, -2, and -3 factors were fused to the human estrogen receptor hormone binding domain.16 When transfected into primary avian erythroid progenitor cells, GATA-1 and GATA-2 chimeras had starkly opposite hormone-dependent effects on erythroid differentiation. A number of similar studies have been published since then generally supporting the notion that each GATA factor differs in activity in a wide variety of distinct cell types, and hence they may not be functionally equivalent in vivo.17 In contrast, in vitro erythroid differentiation of GATA-1–deficient ES cells was partially restored by expressing various GATA factors, including a single zinc finger alone.18 19
To resolve the fundamental question of whether GATA-1 possesses unique functional properties that are distinct from the other hematopoietic GATA factors, or whether its spatiotemporal patterning and abundance is the primary requirement for effective erythropoiesis, we attempted to rescue the GATA-1.05 mutant embryonic lethality in vivo by expressing different murine GATA factors.
Materials and methods
Generation of transgenic mice
Transgenic mice were generated by microinjection of DNAs into fertilized BDF1 eggs using standard procedures.20 Founders were first screened by polymerase chain reaction (PCR) and then verified by Southern blot analysis. The copy number of each line was determined using a BAS 1500 Mac imaging analyzer (Fuji Film, Tokyo, Japan). Mice bearing the GATA-1.05 germline mutant allele8 were bred in clean rooms in the Animal Research Center at the University of Tsukuba. To rescue GATA-1.05 mice from embryonic lethality, GATA-1.05 heterozygous female mice were intercrossed with transgene-positive heterozygous stud mice, and progenies from these matings were analyzed at the indicated times.
Genotyping of mice
Genomic DNA was analyzed by PCR using the following primers.Neo gene primers were used for detecting the GATA-1.05allele, because the mutation was generated by inserting a Neogene cassette into the 5′ flanking region of the GATA-1locus.8 Primers for GATA-1, -2, and -3 were used for detecting the respective transgenes.15 We determined the sex of transgenic embryos using PCR amplification of the Y chromosome-specific Zfy-1 gene.21 Southern blots were performed on the same genomic DNA samples. We used full-length complementary DNAs (cDNAs) of GATA-1 and GATA-3 and used GATA-2 cDNA without the finger region as probes.
RNA blot analysis
RNA blot analysis was performed as previously described.6 Total cellular RNA was extracted from spleens of 3- to 6-month-old mice using RNA extraction reagent, RNAzol (Tel-Test, Friendswood, TX). RNA samples (10 μg) were electrophresed on a 1.6% agarose gel and then transferred to nylon membranes (Zeta-probe, Bio-Rad, Hercules, CA). Full-length cDNAs for each GATA factor were used as probes.
Western blot analysis
Electrophoretic mobility shift analysis
The double-stranded GATA oligonucleotide 5′-GCTGATTCCCTTATCTATGCCT TCCCAGCTGCCTCCCT-3′7 was radiolabeled as probe. For competition experiments, a GATA mutant oligonucleotide 5′-GCTGATTCCCTGGCTTATGCCTTCCCAGCTGCCTCCCT-3′ was included. Binding reactions and electrophoresis were carried out as previously described.1
In vitro colony assays
Neonatal spleens were dispersed into single-cell suspensions and plated in duplicate in 1.0% methylcellulose in alpha–minimal essential medium containing 30% fetal calf serum, 50-μM 2-mercaptoethanol, 1% bovine serum albumin (Sigma Chemical Co, St Louis, MO), and Nutridoma-SP (Boerhringer Mannheim, Mannheim, Germany). A total of 2-U/mL human erythropoietin, 50-ng/mL murine stem cell factor, 50-ng/mL human thrombopoietin, 10-ng/mL murine interleukin-3, 10-ng/mL murine granulocyte-macrophage colony-stimulating factor (GM-CSF), and 10-ng/mL murine interleukin-6 were added as supplements. Cultures were maintained at 37°C under humidified conditions with 5% carbon dioxide. Colony-forming units–erythroid (CFU-E) were counted on day 3, and burst-forming units–erythroid (BFU-E), CFU-GM, and CFU-megakaryocytes (CFU-MEG) were scored on day 7.
Peripheral blood cell analysis
Mice were bled from the retroorbital plexus, and blood cell indices were determined by hemocytometer (MEK-6258, Nihon Koden Co, Tokyo, Japan). Peripheral blood smears were stained with Wright-Giemsa stain.
Statistical analysis
Statistical analysis was performed using the Studentt test.
Results
Generation of IE3.9int-directed GATA transgenic mice
As diagrammed in Figure 1A, the GATA-1, -2, -3 or mutated GATA-1 (GATA-1 delta C-finger) cDNA was individually cloned 3′ of a GATA-1 genomic fragment that is capable of conferring the complete GATA-1 expression profile to a reporter transgene, and the resultant constructs were injected into fertilized eggs to generate transgenic mice. To monitor the cell types that expressed the transgene-derived GATA factors, in 1 experiment we coinjected a green fluorescent protein (GFP) cDNA placed under IE3.9int transcriptional control.
Transgenic mice bearing IE3.9int-directed transgenes.
(A) Structure of the GATA-1 gene regulatory region (IE3.9int) cassette. This plasmid contains 3.9 kilobase pairs of sequences 5′ to the IE exon, the IE exon itself, the first intron, and a part of the second exon of the mouse GATA-1gene. The initiation methionine codon in the GATA-1 second exon was deleted and replaced by a unique NotI site for subsequent cloning purpose. Restriction enzyme sites are B,BamHI; E, EcoRI; N, NotI; S,SacI. (B-D) Genomic Southern blot analyses ofIE3.9int-directed transgenic mice. The transgene-specific bands (Tg) and endogenous bands(s) (end) are indicated by arrows on the left of each panel. (E, F) Expression profiles of the IE3.9int-GFPtransgene in bone marrow hematopoietic cells. Strong green fluorescence is observed in both erythroid cells and megakaryocytes (arrow) (E). In FACS analysis, most TER119-positive bone marrow cells are also GFP-positive (F).
Transgenic mice bearing IE3.9int-directed transgenes.
(A) Structure of the GATA-1 gene regulatory region (IE3.9int) cassette. This plasmid contains 3.9 kilobase pairs of sequences 5′ to the IE exon, the IE exon itself, the first intron, and a part of the second exon of the mouse GATA-1gene. The initiation methionine codon in the GATA-1 second exon was deleted and replaced by a unique NotI site for subsequent cloning purpose. Restriction enzyme sites are B,BamHI; E, EcoRI; N, NotI; S,SacI. (B-D) Genomic Southern blot analyses ofIE3.9int-directed transgenic mice. The transgene-specific bands (Tg) and endogenous bands(s) (end) are indicated by arrows on the left of each panel. (E, F) Expression profiles of the IE3.9int-GFPtransgene in bone marrow hematopoietic cells. Strong green fluorescence is observed in both erythroid cells and megakaryocytes (arrow) (E). In FACS analysis, most TER119-positive bone marrow cells are also GFP-positive (F).
After screening, 4 independent transgenic lines bearing the GATA-1 cDNA, 3 lines with the mutated GATA-1 cDNA, 3 lines carrying GATA-2 and GFP cDNAs and, finally, 4 lines harboring the GATA-3 transgene were generated. The GATA-1 cDNA transgenic lines contained between 1 and 4 copies of the transgene (Figure 1B). Similarly, the GATA-2 and GATA-3 transgenic lines contained 3 to 4 and 4 to 13 transgene copies, respectively (Figure 1C, D). In addition, the “fingerless” GATA-1 cDNA transgenic lines contained 7 (line No. 312) or 2 (line No. 315) transgene copies (data not shown). We employed multiple transgenic lines, which varied in copy numbers and expression levels (verified by reverse transcriptase-PCR) in rescue experiments. Only progeny from F1 or later generations were used in intercross experiments.
Because we coinjected the GATA-2 and GFP expression constructs, which were under the control of identical GATA-1 regulatory sequences, cells that expressed GATA-2 would also be expected to emit green fluorescence. Figure 1E shows a typical bone marrow preparation from such a GATA-2/GFP transgenic mouse. Both megakaryocyte and numerous erythroid cells emitted intense green fluorescence. Total bone marrow cells recovered from a GATA-2/GFP transgenic mouse were reacted with rhodamine-conjugated TER119 antibody and then analyzed by fluorescence-activated flow cytometry (FACS). Figure 1F shows that many TER119-positive cells were also positive for GFP. Because normal bone marrow did not contain any GFP-positive cells (data not shown), these results demonstrated that the GATA-1 regulatory sequences examined here reflect the expression profile of the endogenousGATA-1 gene in vivo. In addition, some cells expressed TER119 but not GFP, suggesting that the GATA-1 gene regulatory sequences in pIE3.9int do not contain a locus control region-like activity that is able to overcome transgene integration position effects.
GATA-1 rescues GATA-1.05 mutant embryonic lethality
To verify that the GATA-1 genomic fragment used in this study was fully sufficient for complete GATA-1 regulation in erythroid cells, we first asked whether a GATA-1 cDNA placed under its regulatory control could abrogate the GATA-1.05 mutant phenotype. We examined 95 pups from 12 litters obtained from 3 independent GATA-1 transgenic lines (Nos. 782, 801, and 831), which contained approximately 2, 4, and 3 transgene copies, respectively, and recovered 11 newborns of compound mutant (G1R) genotype (Table1A). The results thus indicate that when GATA-1 is expressed under the transcriptional control of the IE3.9int genomic sequence, GATA-1.05 transgene-positive male embryos can overcome the lethality due to the germline mutation. A similar phenotypic rescue was not observed in compound mutant male neonates bearing a truncated GATA-1 cDNA, which had the carboxyl finger (C-finger) of GATA-1 deleted (Table 1B); nor was rescue evident during embryogenesis at E13.5 (data not shown).
GATA-2 and GATA-3 can substitute for GATA-1 in erythropoiesis in vivo
To ascertain whether heterotypic hematopoietic GATA factors could functionally replace GATA-1 in erythropoiesis, we generated transgenic lines expressing either GATA-2 or GATA-3 cDNA under GATA-1 gene transcriptional control. Because GATA-1.05 hemizygous males do not survive beyond E12.5,8 we looked for preliminary evidence of rescue at E13.5. Figure 2A shows representative genotyping results of offspring from an intercross between a GATA-1.05/X female and an IE3.9int–GATA-2transgenic male (line No. 620, which had 4 transgene copies). Significantly, embryo No. 4, which was positive for all 3 (Neo,Zfy-1, and GATA-2 transgene) markers, was still alive and displayed grossly normal morphology at E13.5 (G2R; Figure 2C).
Rescue of hematopoiesis in GATA-1.05 mouse by transgenic expression of GATA-2.
(A) Genotyping of GATA-1.05::GATA-2 Tgcompound mutant mice. Three sets of primers were used for the genotyping. Primer sets for the neomycin resistance gene (Neo) and Zfy-1 gene were used for detecting GATA-1.05 allele and sex of the embryos, respectively. Another set of primers was used to detect the GATA-2 transgene. Numbers represent sibling embryos from a single litter. (B-D) Gross appearance of a litter of E13.5 embryos from the mating of GATA-1.05 heterozygous female mouse with GATA-2 Tg(+) male mouse. An embryo withGATA-1.05/Zfy-1(+)/GATA-2 Tg(–) genotype was found dead at E13.5 and showed signs of necrosis (B). In contrast, an embryo ofGATA-1.05(+)/Zfy-1(+)/GATA-2 Tg(+) genotype was apparently healthy (G2R mouse; C), albeit slightly smaller in size, compared with WT male embryo (D).
Rescue of hematopoiesis in GATA-1.05 mouse by transgenic expression of GATA-2.
(A) Genotyping of GATA-1.05::GATA-2 Tgcompound mutant mice. Three sets of primers were used for the genotyping. Primer sets for the neomycin resistance gene (Neo) and Zfy-1 gene were used for detecting GATA-1.05 allele and sex of the embryos, respectively. Another set of primers was used to detect the GATA-2 transgene. Numbers represent sibling embryos from a single litter. (B-D) Gross appearance of a litter of E13.5 embryos from the mating of GATA-1.05 heterozygous female mouse with GATA-2 Tg(+) male mouse. An embryo withGATA-1.05/Zfy-1(+)/GATA-2 Tg(–) genotype was found dead at E13.5 and showed signs of necrosis (B). In contrast, an embryo ofGATA-1.05(+)/Zfy-1(+)/GATA-2 Tg(+) genotype was apparently healthy (G2R mouse; C), albeit slightly smaller in size, compared with WT male embryo (D).
In the absence of the GATA-2 transgene, GATA-1.05 hemizygous male embryos did not survive beyond E12.5 (Figure 2B) and exhibited significant necrosis by E13.5. We analyzed 8 litters of 46 embryos between gestational days 13.5 and 16.5 (data not shown) as well as 75 newborns from 12 litters using GATA-2–expressing transgenic line No. 620 (Table 2) and found that all live males had the GATA-2 transgene. As expected, no transgene-negativeGATA-1.05 male was recovered at birth. Similar results were observed when 2 other GATA-2 transgenic lines (No. 625, 3 copies; No. 99, 4 copies; Table 2) were used in similar breeding paradigms. These data indicated that GATA-2, when expressed under GATA-1 transcriptional control, could rescue the GATA-1.05 mutation.
We next asked whether a GATA-3 transgene could similarly overcome the mid embryonic lethal effect of the GATA-1.05 mutation. Unlike GATA-1 and GATA-2, GATA-3 is not expressed in erythroid or megakaryocytic lineage cells at significant levels; therefore, this experiment would constitute a cardinal test for possible functional redundancy among the hematopoietic GATA family members. Two independent GATA-3 transgenic lines (Nos. 390 and 820, bearing approximately 13 and 9 transgene copies, respectively) were mated with GATA-1.05heterozygous female mice. Significantly, both GATA-3 transgenic lines were able to rescue GATA-1.05 hemizygotic males (GATA-3; Table2). These results unequivocally demonstrated that GATA-2 and GATA-3, when expressed under the regulatory control of the GATA-1locus, could overcome the lethal phenotype caused by the GATA-1 germline loss of function mutation.
Compound mutant mice express transgene-derived GATA proteins
To determine the expression levels of transgene-derived GATA factors in these rescued animals, RNA blot analysis was performed on RNAs prepared from G1R, G2R, and G3R transgenic spleens. Transcripts derived from the GATA-1, -2, or -3 transgenes were abundant in the spleens of all compound mutant mice (Figure 3A). As expected, endogenous GATA-1 RNA (G1-end) was not detected in the G2R or G3R mice.
Expression of transgene in each rescued mouse.
(A) RNA blot analyses of rescued GATA-1.05 compound mutant mice. Total cellular RNA was extracted from the spleens of G1R (line No. 782), G2R (line No. 620), G3R (line No. 390), or WT mice. The transcript sizes of endogenous and transgenic RNA transcripts are as follows: endogenous GATA-1 (G1-end) is 1.9 kilobases (kb); transgene (G1 Tg) of GATA-1 is 2.1 kb; endogenous GATA-2 (G2-end) is 3.5 and 2.9 kb; transgene (G2 Tg) of GATA-2 is 2.3 kb; transgene of GATA-3 (G3 Tg) is 2.7 kb. A β-actin probe was used as the internal control. (B, C) Western blot analysis of G1R or G2R lines. To examine protein levels of transgene-derived GATA factors, splenic nuclear extracts prepared from various transgenic lines (indicated above the lanes) were subjected to Western blot analysis using anti–GATA-1 N6 monoclonal antibody (B) or anti–GATA-2 RC1.1 monoclonal antibody (C). For controls, nuclear extract from MEL cells (+) or IE3.9int-GFP mouse spleen was used. (D) EMSA of transgenic GATA factors. Radiolabeled GATA probe was incubated without (lane 1) or with splenic nuclear extracts from a WT mouse (lanes 2-3), G1R line No. 782 (lanes 4-6), G2R line No. 620 (lanes 7-9), and G3R line No. 390 (lanes 10-12) in the presence of cold GATA (lanes 3, 5, 8, 11) or mutated GATA (lanes 6, 9, 12) oligonucleotides. G1R and G3R were similar to WT, but G2R was significantly higher than WT.
Expression of transgene in each rescued mouse.
(A) RNA blot analyses of rescued GATA-1.05 compound mutant mice. Total cellular RNA was extracted from the spleens of G1R (line No. 782), G2R (line No. 620), G3R (line No. 390), or WT mice. The transcript sizes of endogenous and transgenic RNA transcripts are as follows: endogenous GATA-1 (G1-end) is 1.9 kilobases (kb); transgene (G1 Tg) of GATA-1 is 2.1 kb; endogenous GATA-2 (G2-end) is 3.5 and 2.9 kb; transgene (G2 Tg) of GATA-2 is 2.3 kb; transgene of GATA-3 (G3 Tg) is 2.7 kb. A β-actin probe was used as the internal control. (B, C) Western blot analysis of G1R or G2R lines. To examine protein levels of transgene-derived GATA factors, splenic nuclear extracts prepared from various transgenic lines (indicated above the lanes) were subjected to Western blot analysis using anti–GATA-1 N6 monoclonal antibody (B) or anti–GATA-2 RC1.1 monoclonal antibody (C). For controls, nuclear extract from MEL cells (+) or IE3.9int-GFP mouse spleen was used. (D) EMSA of transgenic GATA factors. Radiolabeled GATA probe was incubated without (lane 1) or with splenic nuclear extracts from a WT mouse (lanes 2-3), G1R line No. 782 (lanes 4-6), G2R line No. 620 (lanes 7-9), and G3R line No. 390 (lanes 10-12) in the presence of cold GATA (lanes 3, 5, 8, 11) or mutated GATA (lanes 6, 9, 12) oligonucleotides. G1R and G3R were similar to WT, but G2R was significantly higher than WT.
To determine the protein level in each transgenic line, we performed Western blot analysis on splenic extracts of each GATA-1 or GATA-2 transgenic line. As evident in Figures 3B and 3C, the protein levels varied among the lines; however, all lines could reverse the lethal phenotype of GATA-1.05 mutation. G2R-line 620 expressed GATA-2 protein more than 20-fold higher than G2R-line 625. In the case of GATA-3 transgenic lines, transgene-derived mRNA levels in both lines were almost identical (data not shown).
The DNA binding activity of the transgene-derived GATA factors was examined by electrophoretic mobility shift analysis (EMSA) using spleen nuclear extracts of the compound G1R, G2R, and G3R mutant animals (Figure 3D). The GATA motif binding activity in G1R or G3R spleens was comparable to that detected in WT spleens (G1R, 146; G3R, 243; and WT, 145 arbitrary densitometric units, respectively). In the case of G2R, the GATA motif binding activity was 10-fold greater (1430 units) than that in the WT spleens, probably because the protein extract was prepared from the highest GATA-2–expressing line, line No. 620 (Figure3C). Because the affinity of all 3 GATA factors for a GATA-1–preferred binding site is similar,24 this experiment demonstrates that the transgene-derived GATA protein was either similar in abundance to (ie, GATA-3) or 10 times more than (ie, GATA-2) endogenous GATA-1 protein levels.
Hematopoietic colony activity in G2R mice
As an independent measure of whether hematopoiesis was normal in the G2R newborns, we conducted in vitro progenitor colony assays. The colony-forming activity of G2R newborns was found to be almost normal when compared with that of WT littermates (Table3). Because we had previously shown that the CFU-E activity of GATA-1.05 hemizygous embryos at E11.5 was less than 5% of that of the WT embryos,8 this result indicated that the CFU-E activity of G2R newborns had been fully restored. Interestingly, there were more BFU-E colonies in the spleen cultures of animals carrying the GATA-2 transgene than in WT newborns (31 ± 5.2 colonies/105 vs 12 ± 6.5 colonies/105 spleen cells, respectively). Thus, the GATA-2 transgene fully restored hematopoietic colony-forming activity in theGATA-1.05 genetic background.
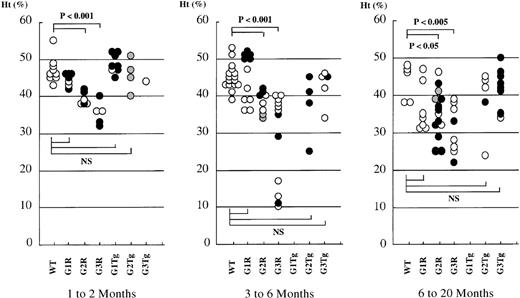
G2R and G3R mice display abnormal adult hematopoiesis
To determine whether the G2R or G3R mice exhibited adult phenotypes, erythropoiesis was examined. While there were no significant differences in the hematocrits of WT mice with or without transgene copies, there was a severe decrease of the hematocrit in GATA-2 and GATA-3 rescued mice. The hematocrits of both compound mutant mice were low, and the severity of the consequent anemia in the G3R was more apparent than in that of G2R mice (Figure4). All rescued mice lived beyond 6 months, and the mean hematocrit values of G2R and G3R were constant over that time. Compared with the peripheral blood smears of G1R mice (Figure5A), G2R and G3R mice displayed markedly abnormal blood cell morphology. In G2R and G3R mice, nucleated red blood cells as well as erythrocytes containing Howell-Jolly bodies and polychromatic erythrocytes were observed (Figure 5B, C). Marked reticulocytosis was observed in G2R and G3R mice (Figure 5E, F). Anisocytosis was detected in scanning electron microscopic analysis of G3R (Figure 5I). Peripheral blood cells from transgenic mice that had no germline mutation displayed normal morphology (data not shown).
Hematocrits of rescued mice.
Hematocrits (Ht) of WT mice carrying no (WT) or GATA-1 (G1 Tg), GATA-2 (G2 Tg), or GATA-3 (G3 Tg) transgenes and depicted alongside that of compound mutant G1R (line No.782, open circle; No. 801, closed circle), G2R (line No. 620, open circle; No. 625, closed circle; No. 99, gray circle), and G3R (line No. 390, open circle; No. 820, closed circle) adult mice. Each dot represents the Ht of an individual mouse. Ht values of 1- to 2-month-old mice, 3- to 6-month-old mice, and 6- to 20-month-old mice are shown.
Hematocrits of rescued mice.
Hematocrits (Ht) of WT mice carrying no (WT) or GATA-1 (G1 Tg), GATA-2 (G2 Tg), or GATA-3 (G3 Tg) transgenes and depicted alongside that of compound mutant G1R (line No.782, open circle; No. 801, closed circle), G2R (line No. 620, open circle; No. 625, closed circle; No. 99, gray circle), and G3R (line No. 390, open circle; No. 820, closed circle) adult mice. Each dot represents the Ht of an individual mouse. Ht values of 1- to 2-month-old mice, 3- to 6-month-old mice, and 6- to 20-month-old mice are shown.
Peripheral blood analysis of rescued GATA-1.05mice.
Blood samples were obtained from 5-week-old mice. While peripheral blood from a G1R line No. 782 mouse (A) contains normal red blood cells, the peripheral blood from G2R line No. 620 (B) and G3R line No. 390 (C) contains nucleated red blood cells. Reticulocytosis is evident in G2R (E) and G3R (F) but not in G1R (D). (G-I) Scanning electron microscopy of peripheral red blood cells. Anisocytosis is observed in G3R (I). Original magnifications in panels G, H, and I are × 10 000, × 2300, and × 4300, respectively.
Peripheral blood analysis of rescued GATA-1.05mice.
Blood samples were obtained from 5-week-old mice. While peripheral blood from a G1R line No. 782 mouse (A) contains normal red blood cells, the peripheral blood from G2R line No. 620 (B) and G3R line No. 390 (C) contains nucleated red blood cells. Reticulocytosis is evident in G2R (E) and G3R (F) but not in G1R (D). (G-I) Scanning electron microscopy of peripheral red blood cells. Anisocytosis is observed in G3R (I). Original magnifications in panels G, H, and I are × 10 000, × 2300, and × 4300, respectively.
Hemocytometric analysis revealed that G2R and G3R animals displayed normocytic and normochromic anemia and thrombocytopenia, although white blood cell counts were similar (Table 4). In addition, there was no significant difference in peripheral blood indices between WT mice with or without GATA transgene(s). These data strongly indicate that the phenotypes observed in GATA-2 or GATA-3 rescued mice are not due to overexpression of the GATA-2 or GATA-3 proteins or to silencing of transgene expression.
Hence, we concluded that the GATA-1 molecule specifies functions in adult erythropoiesis that cannot be completely complemented by the GATA-2 or GATA-3 proteins. The nature of these functions remains to be elucidated.
Discussion
In this study, we comprehensively evaluated the efficacy of substituting other hematopoietic GATA factors for the erythroid functions of GATA-1 in vivo. G1R compound mutant males do not succumb to embryonic lethality as do GATA-1.05 mutant males. This result provides compelling evidence that the IE3.9int genomic fragment contains sequences that can elicit proper physiologic levels ofGATA-1 gene transcription. To our surprise, GATA-2 and GATA-3 transgenes could also fully compensate for the loss of GATA-1 function in utero, resulting in normal embryonic and perinatal hematopoiesis. These data thus illustrate that while precise transcriptional regulation of the GATA-1 gene is vital for normal erythropoiesis, the identity of the GATA factor is inconsequential for prenatal hematopoiesis.
Because we employed a GATA-1 knockdown mutant allele that retained only 5% of the WT GATA-1 mRNA levels, it remained formally possible that residual endogenous GATA-1 activity could taint our conclusions. However, based on the following lines of evidence, we believe this to be unlikely. First, there was no induction above the undetectable level of endogenous GATA-1 transcripts in the G2R or G3R compound mutant mice. Second, GATA-1 was not detectable by immunostaining in the fetal livers of G2R compound mutant embryos (data not shown). Third, the GATA-1.05 mutant phenotype was not rescued by the expression of a modified GATA-1 transgene that was missing the C-finger, which is required for site-specific DNA binding. Thus, the rescue of the GATA-1.05 germline mutation is absolutely dependent on the expression of functional, DNA binding, transgene-derived GATA factor, and the residual level of endogenous GATA-1 in the GATA-1.05 mutant background never became empirically significant.
Several precedents illustrate that related transcription factors are often functionally interchangeable in vivo. Knock-in experiments in the mouse have shown that myogenin can replace Myf5 in rib cage development and that En-2 can replace En-1 in midbrain and hindbrain development.25 26 These studies indicate that each locus has acquired distinctive gene transcriptional regulatory modules that are responsible for eliciting the unique spatial and temporal expression profiles, and it is this attribute that makes each of these factors indispensable in vivo.
In contrast to the present report, a human GATA-3 cDNA inserted into the mouse GATA-1 locus showed incomplete rescue of the GATA-1–null lethal phenotype.27 Consistent with this result, a murine GATA-2 cDNA inserted into the mouse GATA-1locus also could not completely rescue erythroid differentiation in an in vitro ES cell differentiation assay (our unpublished observations). We suggest that the molecular basis for the partial rescue in these experiments is probably complex. It may be due, at least in part, to insufficient accumulation of gene-targeted GATA protein compared with the normal level of GATA-1. Indeed, we showed here that GATA-2 and GATA-3 could rescue the GATA-1 knockdown mouse from embryonic lethality when they were expressed under the regulatory influence of theGATA-1 gene.
It should be noted that in the transgenic approach employed here, the protein levels of transgenic GATA-2 and GATA-3 were similar to or higher than the level of GATA-1 in WT erythroid cells. These results suggest that GATA-2 and GATA-3 proteins or mRNAs may be subject to more stringent posttranslational regulation than is GATA-1 in erythroid cells. If so, a gene-targeted replacement strategy may not always be most suitable for comparing the biological functions of related factors in vivo.
The relative stability of GATA-1, -2, and -3 in erythroid cells may well have biological significance. The mice rescued with the GATA-2 and -3 transgenes suffered from anemia and abnormal hematopoiesis in adulthood. Considering that, in the hematopoietic cells of these transgenic mice, GATA-2 and GATA-3 were expressed at levels similar to or higher than that of endogenous GATA-1 in WT mice and that the mean hematocrit value of older G2R and G3R mice were constant during their foreshortened lifespans, the anemic phenotype in adult animals is probably a consequence of the distinct biochemical natures of the individual GATA proteins.
In summary, transgenic expression of GATA-2 and GATA-3 underGATA-1 transcriptional regulatory influence indeed rescued theGATA-1.05 mutant mouse from embryonic lethality, indicating that precise transcriptional regulation of the GATA-1 gene is most critical for its function in erythropoiesis. The assay system employed here is immediately applicable to the analysis of other transcription factor families and may prove to be especially useful for evaluating the genuine contributions of presumed redundancy among different family members and for revealing subtle functional differences among related members of protein families.
Acknowledgments
We would like to thank Drs Noriko Kajiwara, Fumihiro Sugiyama, Naomi Kaneko, and Kenichi Yagami for help and discussion.
Supported in part by grants-in-aid from the Ministry of Education, Science, Sports and Culture, the Japanese Society for Promotion of Sciences (RFTF), Core Research for Evolutional Sciences and Technology, and the National Institutes of Health (GM 28 896).
Reprints:Masayuki Yamamoto, Center for TARA and Institute of Basic Medical Institute, University of Tsukuba, 1-1-1 Tennodai, Tsukuba 305-8577, Japan; e-mail: masi@tara.tsukuba.ac.jp.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.