Abstract
One mechanism for disrupting the MLL gene in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) is through partial tandem duplication (MLL-PTD); however, the mechanism by which MLL-PTD contributes to MDS and AML development and maintenance is currently unknown. Herein, we investigated hematopoietic stem/progenitor cell (HSPC) phenotypes of Mll-PTD knock-in mice. Although HSPCs (Lin−Sca1+Kit+ (LSK)/SLAM+ and LSK) in MllPTD/WT mice are reduced in absolute number in steady state because of increased apoptosis, they have a proliferative advantage in colony replating assays, CFU-spleen assays, and competitive transplantation assays over wild-type HSPCs. The MllPTD/WT-derived phenotypic short-term (ST)–HSCs/multipotent progenitors and granulocyte/macrophage progenitors have self-renewal capability, rescuing hematopoiesis by giving rise to long-term repopulating cells in recipient mice with an unexpected myeloid differentiation blockade and lymphoid-lineage bias. However, MllPTD/WT HSPCs never develop leukemia in primary or recipient mice, suggesting that additional genetic and/or epigenetic defects are necessary for full leukemogenic transformation. Thus, the Mll-PTD aberrantly alters HSPCs, enhances self-renewal, causes lineage bias, and blocks myeloid differentiation. These findings provide a framework by which we can ascertain the underlying pathogenic role of MLL-PTD in the clonal evolution of human leukemia, which should facilitate improved therapies and patient outcomes.
Introduction
Hematopoiesis requires coordinate changes in gene expression to control the process of cell fates for self-renewal, differentiation, apoptosis, and maturation. Dysregulation of transcriptional controls in hematopoiesis contributes to myelodysplastic syndrome (MDS) and acute myelogenous leukemia (AML). Recurrent chromosomal translocations and gene mutations are found in MDS and AML that affect transcriptional regulators, including transcription factors and epigenetic regulators, such as RUNX1, CBFβ, MLL, and EZH2.1-4 A significant fraction of MDS and MDS/AML have RUNX1 mutations or MLL partial tandem duplication (MLL-PTD),5-8 whereas nearly half of all AML cases are associated with aberrations of MLL or CBF genes.9-11
The Mixed-Lineage Leukemia (MLL) gene was isolated as a common target of chromosomal translocations occurring at 11q23.9,10 These translocations fuse MLL with more than 70 different partner genes. The SET domain of MLL has histone methyltransferase activity that specifically methylates lysine 4 on histone H3 (H3K4), a modification typically associated with transcriptionally active regions of chromatin.12,13 The MLL-PTD was first observed in de novo AML with a normal karyotype or trisomy 11.14-16 Cloning of this region revealed partial duplications within the 5′ region of the MLL gene. These duplications consist of an in-frame repetition of MLL exons in a 5′ to 3′ direction and produce an elongated protein.15 The incidence of MLL-PTD was 5.4% in one study of 956 unselected cases of AML, whereas another series detected MLL-PTD in 6.4% of 988 unselected adult and childhood AML.17,18 All studies confirmed that MLL-PTD is predominantly found in cytogenetically normal AML or in AML with trisomy 11 as a sole cytogenetic abnormality. In several but not all studies, patients with MLL-PTD had shorter disease-free survival and overall survival rates, although only the shortened disease-free survival rate was statistically significant.17-19
How MLL-PTD contributes to MDS or AML is unclear. Two observations reveal potential mechanisms whereby MLL-PTD may disturb normal hematopoiesis: (1) repetitive DNA-binding domains (AT hooks and CXXC domain), present in MLL-PTD, exhibit transactivation potential in vitro20 ; and (2) the release of wild-type (WT) MLL gene suppression in MLL-PTD-positive AML by inhibitors of DNA methyltransferase and histone deacetylase that induce AML blast apoptosis.21 These 2 findings suggest that the MLL-PTD pathway(s) of leukemic transformation are different from those resulting from the fusion of MLL-fused with partners other than itself. Consistent with this notion is the observation that gene expression profiling separates the MLL-PTD AML from those AML containing chimeric MLL gene fusions.22 Thus, in vitro and in vivo modeling is needed to address the specific genetic and epigenetic changes associated with MLL-PTD AML.
We generated a Mll-PTD knock-in mouse model in which its expression is regulated by endogenous promoter, to study the function of Mll-PTD in vitro and in vivo and to identify its downstream targets. Although the MllPTD/WT mice do not develop leukemia, they provide a powerful genetic tool to identify disruptions in normal cellular regulation as a result of this mutation, as well as a model to characterize the contribution of the Mll-PTD in leukemogenesis.23,24 Our initial studies characterizing the MllPTD/WT mice showed that pathways involving myeloid progenitor self-renewal and proliferation are disrupted as a direct result of the Mll-PTD.24 However, a functional dissection of its effect on phenotypically well-defined hematopoietic stem and progenitor cell (HSPC) populations from the MllPTD/WT mice has thus far not been performed. In this report, we further analyzed hematopoiesis in the Mll-PTD knock-in mouse with a specific focus on the function of HSPCs.
Methods
Mice
The MllPTD/WT knock-in mice (CD45.2) were previously described.24 B6.SJL (CD45.1) mice were obtained from the CCHMC/CBDI mouse core. C57Bl/6 × B6.SJL-F1 (CD45.1/CD45.2) were bred in house. All animals were housed in the animal barrier facility at Cincinnati Children's Hospital Medical Center. All animal studies were conducted according to an approved Institutional Animal Care and Use Committee protocol and federal regulations.
Single-cell culture and differentiation assessment
The method of single-cell culture was described previously.25 Briefly, a round-bottom 96-well plate (BD Biosciences) was used for cell culture. Each well contained 200 μL IMDM (Mediatech) supplemented with cytokines (20 ng/mL murine stem cell factor (mSCF) and 20 ng/mL murine thrombopoietin (mTPO), both from PeproTech; 20 ng/mL human G-CSF, 10 ng/mL mIL-3, Peprotech; and 4 U/mL human erythropoietin). Automated deposition of Lin−c-Kit+Sca1+CD150+CD48− cells (LSK/SLAM+) or Lin−c-Kit+Sca1+CD150+or −CD48+ cells (LSK/SLAM−) or granulocyte-macrophage progenitor (Lin−c-kit+Sca1−CD34+CD16/32hi; GMP) single cell was carried out by a FACSAria sorter. After cell sorting, the presence of one cell per well was verified under an inverted microscope (Olympus CKX31). The cells were incubated at 37°C in a humidified atmosphere with 5% CO2 in air. Final evaluation of cell division and colony formation was conducted at day 14 of culture. Cytospin slides prepared from the colonies were stained with Camco Stain Pak (Cambridge Diagnostic Inc) according to the manufacturer's suggested procedures. Slides were analyzed on a Motic BA310 microscope system (Motic Inc).
5-FU treatment and HSC analysis
A single dose (150 mg/kg) of 5-fluorouracil (5-FU; Sigma-Aldrich) was administered intraperitoneally into MllPTD/WT or WT mice (4-8 per group). Peripheral blood (PB) counts were measured every 3-4 days. BM cells were collected at different time points and were stained for LSK, CD150, and CD48 antibodies followed by FACS analysis.
Statistical analysis
The Student t test was used to determine the statistical differences between experimental and control groups.
Full methods for flow cytometry, spleen colony-formation assay, BM transplantation assay, CFU assay, and Western blotting are available in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
BM content of HSPCs is reduced in number in MllPTD/WT mice
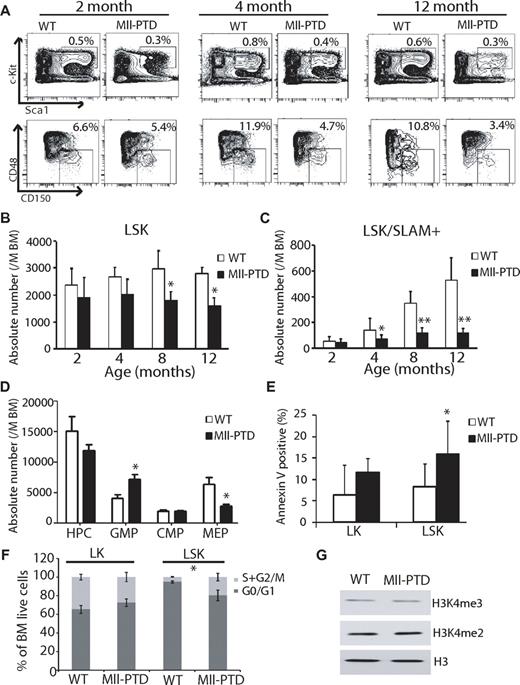
We analyzed the hematopoiesis of MllPTD/WT mice with a specific focus on the function of HSPCs, including LSK, LSK/SLAM, long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), multipotent progenitors (Lin−c-Kit+Sca1+CD34+CD135+; MPPs), and hematopoietic progenitor cells (Lin−c-Kit+Sca1−; HPCs). Consistent with our original report,23 no significant changes in specific blood lineages were observed in MllPTD/WT mice, in either total BM cellularity or PB counts (data not shown). However, the MllPTD/WT mice had significantly reduced numbers of immunophentoypically defined LSKs at 8 and 12 months of age and LSK/SLAM+ populations in the BM at 4, 8, and 12 months of age compared with their age- and sex-matched WT littermate controls (Figure 1A-C). In MllPTD/WT mice, we found similar trends for reduction in LSKs and LSK/SLAM+ populations in the spleen and PB at 4, 8, and 12 months of age compared with their age- and sex-matched WT littermate controls (data not shown). MllPTD/WT mice exhibit an increase in the GMP population at the expense of the megakaryocyte-erythroid progenitor cells (Lin−c-Kit+Sca1− CD34−CD16/32low; MEP) population (Figure 1D). To determine the cellular mechanism responsible for these effects, we analyzed BM cells for apoptotic and cell cycle changes at steady-state hematopoiesis. There was a significant increase in apoptosis of the LSK population of MllPTD/WT mice and a trend toward increased apoptosis in the LK population (Figure 1E). The MllPTD/WT LSK population showed a significant increase in S + G2/M phase but reduction in G0/G1 phase compared with WT controls (Figure 1F). There was a modest (not significant) reduction of S + G2/M phase for the MllPTD/WT LK population. Our data suggest that the increased rate of apoptosis may partially explain the loss of the LSK and LSK/SLAM+ populations in MllPTD/WT mice. MLL is involved in epigenetic regulation of H3K4 methylation. It is still unclear how MLL-PTD affects MLL methyltransferase activity, which is still present in the PTD allele. We purified HSPCs from MllPTD/WT and WT controls and found that there is no global change in H3K4me3 and H3K4me2 methylation levels (Figure 1G). Thus, the function of MllPTD/WT could be locus-specific.
BM content of HSPCs is reduced in number in MllPTD/WTmice. (A) BM of age- and sex-matched littermate WT controls and MllPTD/WT mice were analyzed at 2, 4, and 12 months of age, based on the immunophenotype analysis. Representative flow cytometry (FACS) contour diagram shows the frequency of LSK and LSK/SLAM+ BM cells of WT and MllPTD/WT mice. Shown is a representative of 8 experiments with similar results. (B) Absolute number of LSK cells of WT and MllPTD/WT mice. MllPTD/WT LSK population is reduced in absolute number during aging (n = 8). The difference between control and MllPTD/WT was significant at 8 and 12 months. *P < .05. (C) Absolute number of LSK/SLAM+ cells of WT and MllPTD/WT mice. MllPTD/WT LSK/SLAM+ populations are reduced in absolute number during aging (n = 8). The difference between control and MllPTD/WT was significant at 4, 8, and 12 months. *P < .05. **P < .01. (D) Absolute number of progenitors HPC, GMP, common myeloid progenitor cells (Lin−c-Kit+Sca1−CD34+CD16/32mid; CMPs), and MEP of WT and MllPTD/WT mice. MllPTD/WT mice GMP population is increased at the expense of the MEP population (2 experiments, n = 8; 4-month-old mice were used). The differences of GMP and MEP between control and MllPTD/WT were significant (P < .05). (E) Apoptosis was checked by annexin V staining. Data are the mean percentage ± SD of annexin V+/7 AAD− and annexin V+/7 AAD+. *P < .05. **P < .01. Experiments were performed in duplicate groups for 4 mice at 4-month-old per genotype repeated in 3 separate experiments. (F) Cell-cycle analysis was performed with a BrdU flow kit. Percentage of cycling cells: G0/G1 and S/G2/M are shown for LK (Lin−Kit+) and LSK fractions (2 experiments, n = 4; 4-month-old mice were used). *P < .05. (G) H3K4 methylation in LSK fractions. BM cells were harvested and LSK cells were selected using autoMACS. Western blots were done using indicated antibodies (anti-H3K4me3, anti-H3K4me2, and anti-H3). Representative data were from 3 independent experiments.
BM content of HSPCs is reduced in number in MllPTD/WTmice. (A) BM of age- and sex-matched littermate WT controls and MllPTD/WT mice were analyzed at 2, 4, and 12 months of age, based on the immunophenotype analysis. Representative flow cytometry (FACS) contour diagram shows the frequency of LSK and LSK/SLAM+ BM cells of WT and MllPTD/WT mice. Shown is a representative of 8 experiments with similar results. (B) Absolute number of LSK cells of WT and MllPTD/WT mice. MllPTD/WT LSK population is reduced in absolute number during aging (n = 8). The difference between control and MllPTD/WT was significant at 8 and 12 months. *P < .05. (C) Absolute number of LSK/SLAM+ cells of WT and MllPTD/WT mice. MllPTD/WT LSK/SLAM+ populations are reduced in absolute number during aging (n = 8). The difference between control and MllPTD/WT was significant at 4, 8, and 12 months. *P < .05. **P < .01. (D) Absolute number of progenitors HPC, GMP, common myeloid progenitor cells (Lin−c-Kit+Sca1−CD34+CD16/32mid; CMPs), and MEP of WT and MllPTD/WT mice. MllPTD/WT mice GMP population is increased at the expense of the MEP population (2 experiments, n = 8; 4-month-old mice were used). The differences of GMP and MEP between control and MllPTD/WT were significant (P < .05). (E) Apoptosis was checked by annexin V staining. Data are the mean percentage ± SD of annexin V+/7 AAD− and annexin V+/7 AAD+. *P < .05. **P < .01. Experiments were performed in duplicate groups for 4 mice at 4-month-old per genotype repeated in 3 separate experiments. (F) Cell-cycle analysis was performed with a BrdU flow kit. Percentage of cycling cells: G0/G1 and S/G2/M are shown for LK (Lin−Kit+) and LSK fractions (2 experiments, n = 4; 4-month-old mice were used). *P < .05. (G) H3K4 methylation in LSK fractions. BM cells were harvested and LSK cells were selected using autoMACS. Western blots were done using indicated antibodies (anti-H3K4me3, anti-H3K4me2, and anti-H3). Representative data were from 3 independent experiments.
Expansion of MllPTD/WTmice HSPCs in CFU-spleen and replating assays
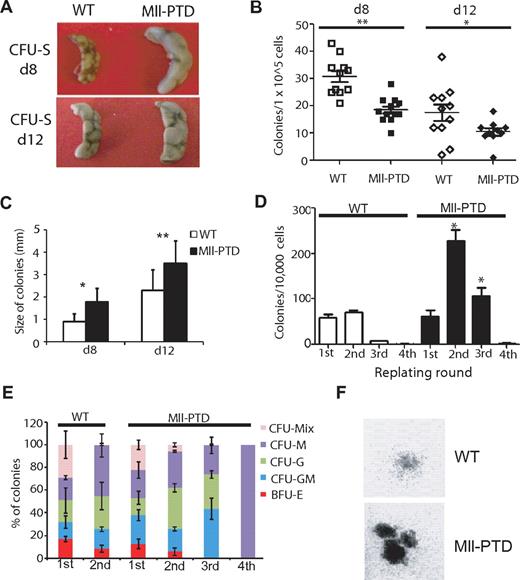
To dissect the function of MllPTD/WT HSPCs, we performed standard in vivo CFU-spleen assays with BM-derived HSPCs from age- and sex-matched littermates of 4-month-old MllPTD/WT and WT controls. We found a 50% reduction in the number of day 8 CFU-spleen and day 12 CFU-spleen colonies derived from MllPTD/WT BM compared with WT BM, indicating reduced numbers of early and committed MllPTD/WT progenitors (Figure 2A-B), consistent with the reduction of phenotypically defined early and committed MllPTD/WT progenitors noted in Figure 1B. CFU-spleen colonies derived from MllPTD/WT mice BM cells were significantly larger than those seen in controls (Figure 2C).
Expansion of MllPTD/WTmice HSPCs in CFU-spleen and replating assays. (A) Representative image of CFU-spleen assay. (B) Number of colonies was counted 8 or 12 days after transplantation (12 mice per group; 1 × 105 cells per mouse). *P < .05. **P < .01. (C) Diameter of each colony was measured under inverted microscope. (D) Frequency of CFU-Cs in the BM of WT and MllPTD/WT during serial replating on methylcellulose in vitro. (E) Proportion of CFU-Cs in serial replating. (F) Representative image of colonies from WT and MllPTD/WT BM cells in the second round of replating.
Expansion of MllPTD/WTmice HSPCs in CFU-spleen and replating assays. (A) Representative image of CFU-spleen assay. (B) Number of colonies was counted 8 or 12 days after transplantation (12 mice per group; 1 × 105 cells per mouse). *P < .05. **P < .01. (C) Diameter of each colony was measured under inverted microscope. (D) Frequency of CFU-Cs in the BM of WT and MllPTD/WT during serial replating on methylcellulose in vitro. (E) Proportion of CFU-Cs in serial replating. (F) Representative image of colonies from WT and MllPTD/WT BM cells in the second round of replating.
We previously found that MllPTD/WT fetal liver cells and adult splenocytes have significantly increased burst forming unit-erythroid, colony-forming unit granulocyte, erythroid, macrophage, megakarycyte (CFU-MIX), and colony-forming unit granulocyte, macrophage (CFU-GM).23,24 We extended our analysis with 4-month-old MllPTD/WT BM cells in a CFU replating assay with M3434 methylcellulose-based medium. We found that MllPTD/WT BM cells produced significantly more colonies in the second and third replating compared with WT BM cells (Figure 2D-E). A significant number of dense CFU-MIX colonies were seen in the first and second replating of the MllPTD/WT BM cells, whereas WT BM cells showed few CFU-MIX clones in the first and rarely in the second replating (Figure 2F). These results indicate that MllPTD/WT BM cells may maintain an immature phenotype longer than the WT BM cells, perhaps because of enhanced self-renewal activity.
MllPTD/WT BM contains an increased number of competitive repopulating LT-HSCs
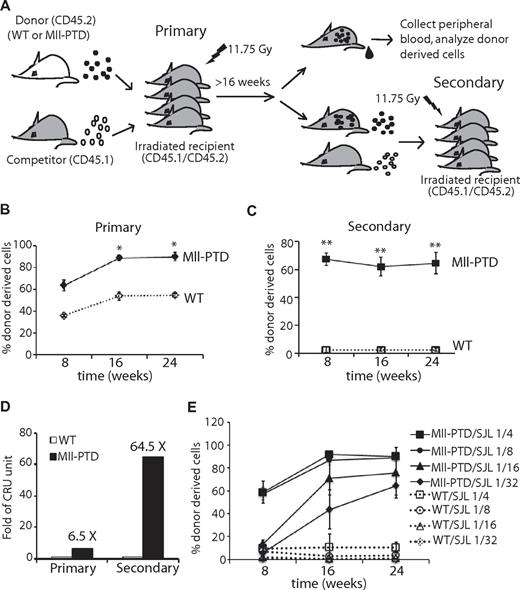
To determine whether MllPTD/WT HSCs exhibit enhanced self-renewal, we next performed standard BM transplantation (BMT) assays to examine the engraftment potential of 4-month-old MllPTD/WT HSPCs in vivo (Figure 3A). We found increased frequencies of donor-derived reconstitution in the CD45.1/CD45.2 recipient mice at a starting 1:1 ratio of MllPTD/WT BM cells (CD45.2) with competitor WT cells (CD45.1; Figure 3B). Up to 90% donor MllPTD/WT CD45.2 chimerism was observed at 16- to 24-week time points in BM (data not shown) and in PB, whereas WT donor CD45.2 cells maintained ∼ 50% chimerism (Figure 3B). We purified the CD45.2 cells from primary recipients at 16 weeks and performed secondary transplantation at a 1:1 ratio with WT competitor cells (CD45.1). The MllPTD/WT derived cells could still maintain high chimerism (up to 60%-70%) in the recipients, whereas mice receiving WT-derived cells demonstrated low chimerism in BM (data not shown) and PB (1%-2%) at 16 weeks (Figure 3C). Calculations showed that MllPTD/WT BM cells have 6.5 times (primary) and 64.5 times (secondary) more competitive repopulating units (CRU) than do age- and sex-matched WT controls (Figure 3D).
Increased competitive repopulating LT-HSCs in MllPTD/WT BM cells. (A) Experimental setup. Lethally irradiated groups of CD45.1+/CD45.2+ WT recipient mice are intravenously injected with 1.5 × 106 BM-MNCs from WT or MllPTD/WT (CD45.2+) mice together with an equal number of CD45.1+ competitor cells. PB is collected from recipients monthly and analyzed by FACS for the presence of CD45.2+ donor-derived cells. Secondary competitive BMT is performed 16 weeks after transplantation, with purified CD45.2+ BM cells from primary recipients together with CD45.1+ competitor cells at a 1:1 ratio. Chimerisms in primary BMT (B) and secondary competitive BMT (C) were assessed monthly. Data shown are the mean percentage ± SD of donor-derived cells (CD45.2+) in PB (n = 8). *P < .05. **P < .01. (D) The competitive repopulation unit (CRU) was calculated 16 weeks after BMT according to the formula: donor RU = % donor × competitor cell number/(100 − % donor), and compared between MllPTD/WT with WT. (E) WT or MllPTD/WT (CD45.2+) donor cells in a serial diluted dose were transplanted into lethally irradiated CD45.1+/CD45.2+ WT recipient mice along with 1 × 105 WT (CD45.1+) helper cells, and the donor engraftment in recipient blood was determined monthly (n = 4).
Increased competitive repopulating LT-HSCs in MllPTD/WT BM cells. (A) Experimental setup. Lethally irradiated groups of CD45.1+/CD45.2+ WT recipient mice are intravenously injected with 1.5 × 106 BM-MNCs from WT or MllPTD/WT (CD45.2+) mice together with an equal number of CD45.1+ competitor cells. PB is collected from recipients monthly and analyzed by FACS for the presence of CD45.2+ donor-derived cells. Secondary competitive BMT is performed 16 weeks after transplantation, with purified CD45.2+ BM cells from primary recipients together with CD45.1+ competitor cells at a 1:1 ratio. Chimerisms in primary BMT (B) and secondary competitive BMT (C) were assessed monthly. Data shown are the mean percentage ± SD of donor-derived cells (CD45.2+) in PB (n = 8). *P < .05. **P < .01. (D) The competitive repopulation unit (CRU) was calculated 16 weeks after BMT according to the formula: donor RU = % donor × competitor cell number/(100 − % donor), and compared between MllPTD/WT with WT. (E) WT or MllPTD/WT (CD45.2+) donor cells in a serial diluted dose were transplanted into lethally irradiated CD45.1+/CD45.2+ WT recipient mice along with 1 × 105 WT (CD45.1+) helper cells, and the donor engraftment in recipient blood was determined monthly (n = 4).
Our data indicate MllPTD/WT BM cells have more functional long-term repopulating cells and these cells maintain their activity on transplantation. To examine these findings in more detail, we performed limiting dilution experiments with MllPTD/WT and WT BM cells. We varied the numbers of WT or MllPTD/WT BM cells (CD45.2) mixed with 100 000 helper/competitor BM cells (CD45.1), transplanted them into recipient mice, and examined donor-derived cells at various time points (Figure 3E). We detected high levels of chimerism (up to 90%) of CD45.2 donor-derived cells from mice transplanted with 1:4 and 1:8 ratios of MllPTD/WT (CD45.2) cells to helper/competitor BM cells, whereas WT control transplanted mice showed the expected low chimerism at these same ratios (10% and 1%, respectively). There were initially low percentages of reconstitution (5%-10%) by MllPTD/WT CD45.2 donor-derived cells at the 1:16 and 1:32 ratios, the MllPTD/WT CD45.2 cells could reach up to 70% and 60% of the PB, respectively, by 6 months (Figure 3E). In contrast, CD45.2 WT BM donor-derived cells were essentially below the limit of detection in transplants with 1:16 and 1:32 ratios of CD45.2 WT cells to helper/competitor CD45.1 WT cells. These data indicate that there is a competitive advantage of MllPTD/WT BM cells in the CFU-spleen and BMT assay, even though MllPTD/WT mice have reduced HSPC numbers in the BM compared with their WT littermate controls. The high reconstitution rates of the 1:16 and 1:32 ratios of MllPTD/WT (CD45.2) cells to WT (CD45.1) cells in the recipient mice after BMT were especially surprising, as there are low numbers of MllPTD/WT LSK cells and minimal MllPTD/WT LSK/SLAM+ cells (LT-HSCs) mixed with WT cells based on our data (Figure 1B-C). Thus, the LSK/SLAM− cells, which contain ST-HSCs and MPPs of the MllPTD/WT mice, might be responsible for the long-term reconstitution activity in our limiting dilution BMT assays.
Increased long-term competitive repopulation induced by MllPTD/WT BM cells is not restricted to phenotypically identified HSCs but also comes from ST-HSCs and myeloid progenitors
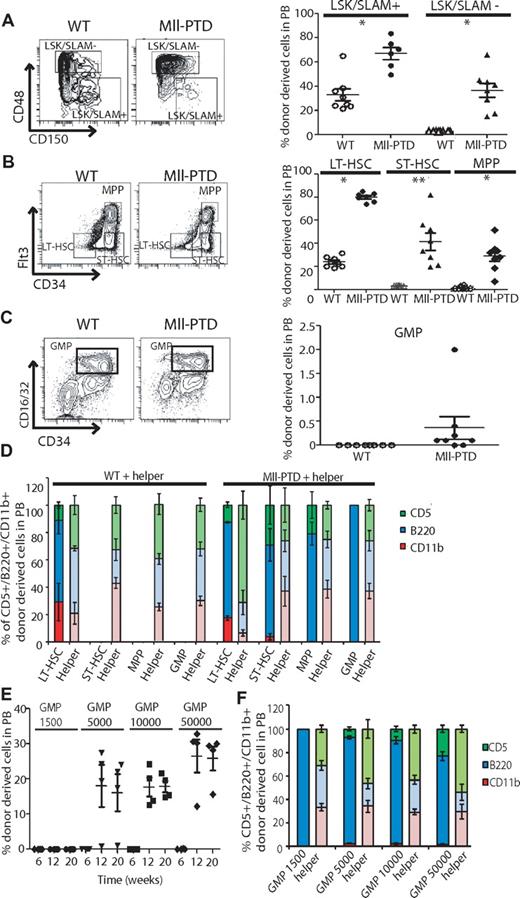
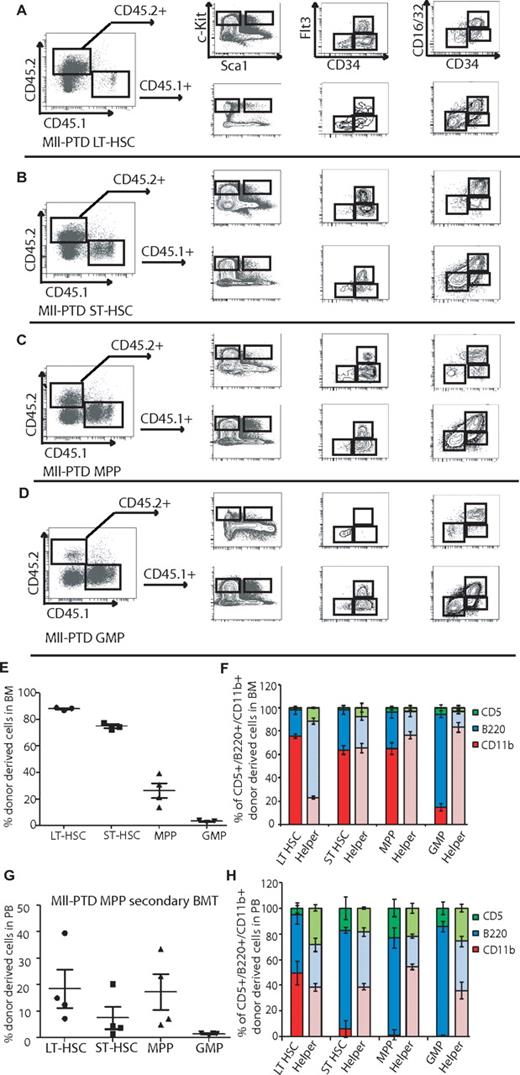
According to the data described in limiting dilution BMT assay, we hypothesized that MllPTD/WT ST-HSCs and/or the HPC populations are responsible for the long-term reconstitution activity in recipient mice. To test it, we performed BMT assays with defined populations of HSPCs sorted on the basis of their cell surface antigen expression. With the well-defined populations of LSK/SLAM+, LSK/SLAM−, or LSK/CD34/Flt3-separated LT-HSCs, ST-HSCs, and MPP populations, we found that LT-HSC–containing populations (LSK/SLAM+ and LSK/CD34−/Flt3−) from MllPTD/WT mice have higher engraftment potential and long-term reconstitution ability than the control WT LT-HSC populations (Figure 4A-B). In WT controls, only LSK/SLAM+ and WT LSK/CD34−/Flt3− populations could reconstitute recipients over the long term (> 4 months). However, the MllPTD/WT ST-HSCs and MPP populations also gave rise to long-term reconstitution (> 8 months). Interestingly, even the MllPTD/WT GMP population is capable of long-term reconstitution (> 4 months), although at a relatively lower level (0.4%). The lower level of reconstitution of MllPTD/WT GMP is in part the result of fewer GMP cells available for the BMT (3500 cells per mouse; Figure 4C). Notably, MllPTD/WT-derived cells in recipient mice exhibited a normal complement of mature lymphoid B and T cells but concurrently had reduced myeloid lineage differentiation. These data are consistent with the association of MLL-PTD with MDS and AML, but not ALL, in human disease. The deficit in myeloid lineage contribution by MllPTD/WT transplanted BM cells was significant (P < .01) in MllPTD/WT LT-HSC recipients, and it was even greater in the MllPTD/WT ST-HSC, MPP, and GMPs BMT recipients (P < .001). There were almost no mature myeloid cells generated from MllPTD/WT MPPs or GMPs in the recipient mice assessed at 4 months after transplantation, whereas their B- and T-cell reconstitution was comparable with helper cell (CD45.1) reconstitution (Figure 4D). A similar myeloid lineage blockade was also found in our limiting dilution BMT assay (Figure 3E; and data not shown). The MPP and GMP BMT are highly lymphoid biased (especially B cells rather than T cells in both PB and BM). We used double-sorted GMPs and performed BMT with dose titration. At 6 weeks after transplantation, we found very low reconstitution with GMP cells (Figure 4E). However, at 12 weeks and 20 weeks, we found significant reconstitution of B cells, T cells, and low percentage of myeloid cells in the PB (Figure 4E-F).
Increased repopulating activity of HSPCs from MllPTD/WT mice is not restricted to phenotypically identified HSCs but also comes from ST-HSCs and myeloid progenitors. The fractions of WT or MllPTD/WT (CD45.2+) BM cells were transplanted into lethally irradiated CD45.1+/CD45.2+ WT recipient mice along with 1 × 105 WT (CD45.1+) helper cells. Engraftment was assessed 16 weeks after transplantation. Shown is the mean ± SD percentage of donor-derived WT or MllPTD/WT cells (CD45.2+) in recipient PB. (A) Equal number of LSK/SLAM+ (3.5 × 102) or LSK/SLAM− (3.5 × 103) fractions from WT or MllPTD/WT BM cells were transplanted into CD45.1+/CD45.2+ WT recipient mice (2 experiments, n = 8). (B) WT or MllPTD/WT (CD45.2+) LT-HSCs (8 × 102) or ST-HSCs (1.2 × 103) or MPP (3.5 × 103) were transplanted into CD45.1+/CD45.2+ WT recipient mice (2 experiments, n = 8). (C) WT or MllPTD/WT (CD45.2+) GMP population cells (3.5 × 103) were transplanted into CD45.1+/CD45.2+ WT recipient mice (2 experiments, n = 8). (D) Frequency of lineage-repopulation myeloid (CD45.2+CD11b+) or B (CD45.2+B220+) or T (CD45.2+CD5+) cells compared with competitor (CD45.1) derived lineage-repopulation present in CD45.1+/CD45.2+ WT recipient mice. *P < .05. **P < .01. (E) MllPTD/WT (CD45.2+) GMP population cells in serial diluted dose (1.5 × 103, 5 × 103, 1 × 104, and 5 × 104) were transplanted into CD45.1+/CD45.2+ WT recipient mice (n = 4). Engraftment was assessed at 6, 12, and 20 weeks after transplantation (n = 4). (F) Frequency of donor-derived lineage-repopulation myeloid or B or T cells compared with competitor (CD45.1) derived lineage-repopulation present in CD45.1+/CD45.2+ WT recipient mice. Data are shown at 12 weeks after transplantation.
Increased repopulating activity of HSPCs from MllPTD/WT mice is not restricted to phenotypically identified HSCs but also comes from ST-HSCs and myeloid progenitors. The fractions of WT or MllPTD/WT (CD45.2+) BM cells were transplanted into lethally irradiated CD45.1+/CD45.2+ WT recipient mice along with 1 × 105 WT (CD45.1+) helper cells. Engraftment was assessed 16 weeks after transplantation. Shown is the mean ± SD percentage of donor-derived WT or MllPTD/WT cells (CD45.2+) in recipient PB. (A) Equal number of LSK/SLAM+ (3.5 × 102) or LSK/SLAM− (3.5 × 103) fractions from WT or MllPTD/WT BM cells were transplanted into CD45.1+/CD45.2+ WT recipient mice (2 experiments, n = 8). (B) WT or MllPTD/WT (CD45.2+) LT-HSCs (8 × 102) or ST-HSCs (1.2 × 103) or MPP (3.5 × 103) were transplanted into CD45.1+/CD45.2+ WT recipient mice (2 experiments, n = 8). (C) WT or MllPTD/WT (CD45.2+) GMP population cells (3.5 × 103) were transplanted into CD45.1+/CD45.2+ WT recipient mice (2 experiments, n = 8). (D) Frequency of lineage-repopulation myeloid (CD45.2+CD11b+) or B (CD45.2+B220+) or T (CD45.2+CD5+) cells compared with competitor (CD45.1) derived lineage-repopulation present in CD45.1+/CD45.2+ WT recipient mice. *P < .05. **P < .01. (E) MllPTD/WT (CD45.2+) GMP population cells in serial diluted dose (1.5 × 103, 5 × 103, 1 × 104, and 5 × 104) were transplanted into CD45.1+/CD45.2+ WT recipient mice (n = 4). Engraftment was assessed at 6, 12, and 20 weeks after transplantation (n = 4). (F) Frequency of donor-derived lineage-repopulation myeloid or B or T cells compared with competitor (CD45.1) derived lineage-repopulation present in CD45.1+/CD45.2+ WT recipient mice. Data are shown at 12 weeks after transplantation.
We also analyzed the BM reconstitution of MllPTD/WT HSPC-derived cells. Although MPP and GMP BMT recipient cells are highly lymphoid biased in PB, they are actually enriched for immunophenotypic GMP populations in the BM of recipient mice. We found similar accumulation of MLL-PTD GMP in the BM of recipients of MLL-PTD LT-HSCs and ST-HSCs (Figure 5A-D). This lymphoid bias could be the result of a blockade to myeloid, but not lymphoid, differentiation. In support of this concept, we found significant myeloid reconstitution in the BM, but not in the PB (Figure 5E-F compared with Figure 4D). We also performed secondary transplantations of MLL-PTD MPP recipient-derived HSPCs and found that they reconstitute secondary recipient mice (Figure 5G-H). Thus, our data suggest that Mll-PTD provides HSPC with self-renewal potential normally restricted to LT-HSCs. Our data indicate that, although HSPCs from MllPTD/WT BM cells can alter differentiation/repopulating properties and generate long-term engraftment potential in progenitor populations, these MllPTD/WT HSPCs have intrinsic defects for myeloid lineage commitment and/or differentiation in vivo.
Phenotypic ST-HSCs, MPP, and GMP from MllPTD/WT mice repopulate LT-HSCs. Representative FACS contour diagram shows the repopulation of LSK, LT-HSC/ST-HSC/MPP, and CMP/GMP/MEP in recipients transplanted with MllPTD/WT different fractions, MllPTD/WT LT-HSCs (A), ST-HSCs (B), MPP (C), and GMP (D). (E) Mean ± SD percentage of donor-derived MllPTD/WT cells (CD45.2+) in recipient BM. (F) Frequency of donor-derived lineage-repopulation myeloid or B or T cells compared with competitor (CD45.1) derived lineage-repopulation present in recipient BM. Data are at 12 weeks after transplantation (n = 4). (G) Secondary transplantation was performed 16 weeks after primary transplantation with sorted fractions from MllPTD/WT MPP transplanted recipients (n = 4). Data are mean ± SD percentage of donor-derived MllPTD/WT cells (CD45.2+) in recipient PB. (H) Frequency of donor-derived lineage-repopulation myeloid or B or T cells compared with competitor (CD45.1) derived lineage-repopulation present in secondary recipient PB 12 weeks after transplantation.
Phenotypic ST-HSCs, MPP, and GMP from MllPTD/WT mice repopulate LT-HSCs. Representative FACS contour diagram shows the repopulation of LSK, LT-HSC/ST-HSC/MPP, and CMP/GMP/MEP in recipients transplanted with MllPTD/WT different fractions, MllPTD/WT LT-HSCs (A), ST-HSCs (B), MPP (C), and GMP (D). (E) Mean ± SD percentage of donor-derived MllPTD/WT cells (CD45.2+) in recipient BM. (F) Frequency of donor-derived lineage-repopulation myeloid or B or T cells compared with competitor (CD45.1) derived lineage-repopulation present in recipient BM. Data are at 12 weeks after transplantation (n = 4). (G) Secondary transplantation was performed 16 weeks after primary transplantation with sorted fractions from MllPTD/WT MPP transplanted recipients (n = 4). Data are mean ± SD percentage of donor-derived MllPTD/WT cells (CD45.2+) in recipient PB. (H) Frequency of donor-derived lineage-repopulation myeloid or B or T cells compared with competitor (CD45.1) derived lineage-repopulation present in secondary recipient PB 12 weeks after transplantation.
Increased repopulating activity of HSPCs from MllPTD/WT mice correlates with acquisition of an intrinsic self-renewal program
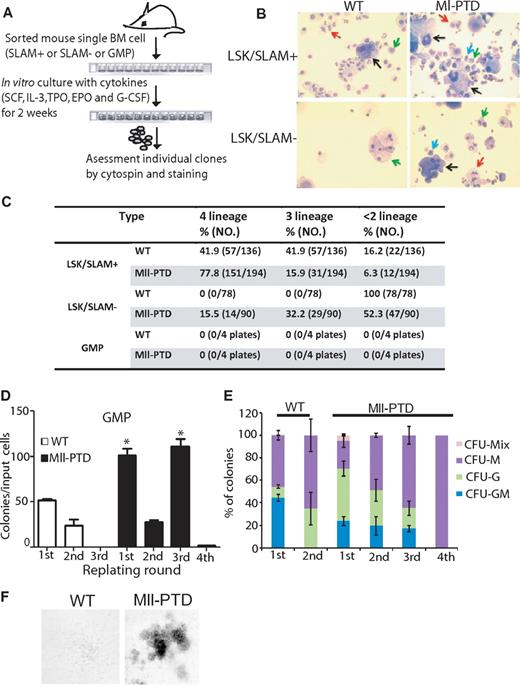
We hypothesized that MllPTD/WT HSPCs have intrinsic altered differentiation/repopulating properties for self-renewal and multilineage differentiation activity, although this activity seems to gradually diminish during the differentiation process, as evidenced by the reduced total reconstitution activity in the mice receiving ST-HSC, MPP, and GMP BM cells (3500 cells/mouse), compared with the mice only receiving 350-800 cells/mouse LT-HSC BM cells (Figure 4B-D). To quantify the multipotent activity of MllPTD/WT individual HSPC subpopulations, we double-sorted single cell of LSK/SLAM+, LSK/SLAM− (which contain both ST-HSCs and MPPs) and GMP populations and performed in vitro culturing in SCF/thrombopoietin/erythropoietin/G-CSF/IL-3–containing liquid cultural medium for 2 weeks and then assessed cell morphology of the resulting individual colonies (Figure 6A-B). In this assay, WT LT-HSCs will generate all 4 lineages of myeloid cells (erythroid, megakaryocyte, granulocyte, and monocyte); however, committed progenitors will give rise to limited lineages.26 WT LSK/SLAM+ BM cells generated 42% tetra-lineage clones, 42% trilineage clones, and 16% bi-lineage or uni-lineage clones. However, LSK/SLAM+ BM cells of MllPTD/WT mice generated 78% tetra-lineage clones, 16% tri-lineage clones, and 6% bi-lineage or uni-lineage clones (P < .001). There was a significant shift toward immaturity of the LSK/SLAM+ population in MllPTD/WT mice. In addition, we found that 15.5% of MllPTD/WT LSK/SLAM− BM cells generated tetra-lineage clones, 32% generated tri-lineage clones, and 52% generated bi-lineage or uni-lineage clones. In contrast, WT LSK/SLAM− BM cells only gave rise to bi-lineage or uni-lineage clones (Figure 6B).
Increased repopulating activity of HSPCs from MllPTD/WT mice correlates with acquisition of an intrinsic self-renewal program. (A) A single-cell culture was performed in the presence of cytokines for 2 weeks. (B) Cytospin slides were prepared from individual clones and stained with Camco Stain Pak. Black arrow indicates megakaryocyte; red arrow, neutrophil; green arrow, monocyte; and blue arrow, poly-erythrocyte. (C) Frequency of lineage formation of LSK/SLAM+ or LSK/SLAM− or GMP in WT or MllPTD/WT are shown in Table 1. (D) Frequency of CFU-Cs in the GMP population sorted from WT or MllPTD/WT during serial replating on methylcellulose in vitro. For the first-round plating, 1 × 103 GMP cells were seeded per milliliter of hematopoietic methylcellulose colony-forming media. A total of 1 × 104 cells were planted for next-round replating. The cells are assayed in triplicate dishes of 1 mL. *P < .05. (E) Proportion of CFU-Cs in serial replating. (F) Representative image of colonies from WT and MllPTD/WT GMP cells in third-round replating.
Increased repopulating activity of HSPCs from MllPTD/WT mice correlates with acquisition of an intrinsic self-renewal program. (A) A single-cell culture was performed in the presence of cytokines for 2 weeks. (B) Cytospin slides were prepared from individual clones and stained with Camco Stain Pak. Black arrow indicates megakaryocyte; red arrow, neutrophil; green arrow, monocyte; and blue arrow, poly-erythrocyte. (C) Frequency of lineage formation of LSK/SLAM+ or LSK/SLAM− or GMP in WT or MllPTD/WT are shown in Table 1. (D) Frequency of CFU-Cs in the GMP population sorted from WT or MllPTD/WT during serial replating on methylcellulose in vitro. For the first-round plating, 1 × 103 GMP cells were seeded per milliliter of hematopoietic methylcellulose colony-forming media. A total of 1 × 104 cells were planted for next-round replating. The cells are assayed in triplicate dishes of 1 mL. *P < .05. (E) Proportion of CFU-Cs in serial replating. (F) Representative image of colonies from WT and MllPTD/WT GMP cells in third-round replating.
We also sorted GMP populations from both WT and MllPTD/WT mice and performed single-cell in vitro cultures under the same conditions. Neither WT- nor MllPTD/WT-derived GMPs showed clonal growth under these culture conditions, which may be the result of the limited number of single cells (four 96-well plates) we analyzed in this assay (Figure 6C). Thus, although 3500 MllPTD/WT GMP cells could give long-term engraftment in vivo, we could not find any multilineage differentiation under the in vitro single-cell experiment conditions. We conclude that the altered differentiation/repopulating property activity of MllPTD/WT HSPCs is reduced as these cells drive toward lineage commitment and differentiation. Alternatively, this in vitro assay may be too stringent and incompatible with cells that have acquired incomplete self-renewal capability. To further address the GMP-altered differentiation/repopulating properties, we sorted BM GMP populations from both WT and MllPTD/WT mice and performed in vitro CFU replating assays. Compared with WT GMPs, MllPTD/WT GMP colonies had significantly greater colony formation in the first replating, but not in the second replating. However, the MllPTD/WT GMP colonies produced colonies on both the third and fourth replating, whereas WT GMP colonies did not (Figure 6D).
The differences between WT and MllPTD/WT colonies are not limited to the clone numbers but also to colony types. The WT GMP cells gave rise to CFU-GM, colony-forming unit-granulocyte (CFU-G), and colony-forming unit macrophage (CFU-M) colonies in the first replating and only gave rise to CFU-G and CFU-M colonies in the second replating. However, the MllPTD/WT GMP cells produced CFU-MIX, CFU-GM, CFU-G, and CFU-M colonies in the first replating and CFU-GM, CFU-G, and CFU-M colonies in the second replating and third replating, In contrast, the fourth replating only yielded a few CFU-M colonies (Figure 6E). We found significant numbers (5%) of dense CFU-MIX colonies in the first replating of the MllPTD/WT GMPs; however, WT GMP cells yielded 0.1% of CFU-MIX colonies in the first replating. Although we found significant numbers of CFU-GM colonies in the second and third replating of the MllPTD/WT GMPs, we did not observe CFU-GM colony in the second replating of WT GMP cells (Figure 6F). These data suggest that MllPTD/WT GMPs are less mature and have enhanced replating activity compared with WT GMPs, despite exhibiting similar differentiation potentials in the in vitro assay. These in vitro observations are also consistent with our in vivo BMT assay that revealed that MllPTD/WT GMP cells have multilineage differentiation potentials and can self-renew.
Rapid expansion and reduced apoptosis of MllPTD/WT LSK/ SLAM+ cells under stress
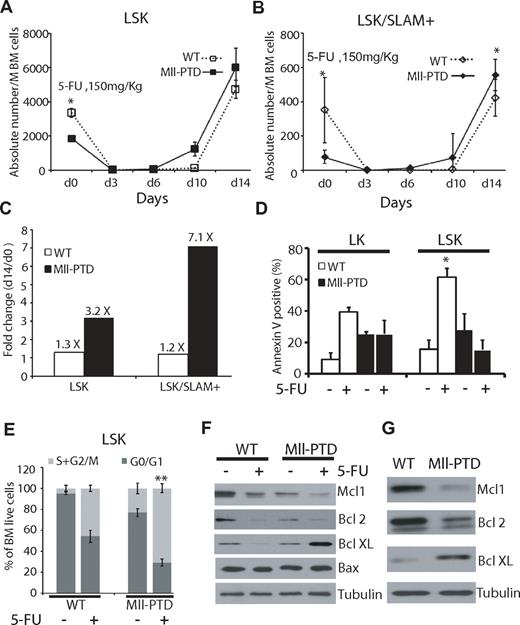
BM cells from MllPTD/WT mice after primary and secondary BMTs exhibited elevated rates of apoptosis at steady state (data not shown). It is known that BMT increases stress on HSPCs, which affects the survival, self-renewal, and proliferation of HSPCs. To evaluate stress-induced signaling in HSPCs (independent of BMT), we treated primary mice with a low dose of 5-FU. We first analyzed PB counts and blood lineages but did not find significant differences between MllPTD/WT and WT groups (data not shown). We further analyzed the HSPCs from the BM of 5-FU-treated mice and found rapid and enhanced expansion of MllPTD/WT LSK versus WT LSK (3.2-fold vs 1.3-fold, P < .01) and MllPTD/WT LSK/SLAM+ versus WT LSK/SLAM+ (7.1-fold vs 1.2-fold, P < .01) at 14 days after 5-FU treatment (Figure 7A-C). These data indicate that, although MllPTD/WT mice have fewer LSK and LSK/SLAM+ populations at steady state, the surviving fraction of HSPCs exhibits increased proliferation after 5-FU exposure. We also analyzed the apoptosis of HSPCs after low-dose 5-FU treatment and found that WT controls have increased apoptosis, but MllPTD/WT LSK BM cells (which have increased apoptosis rate without 5-FU treatment) exhibit reduced apoptosis compared with their WT counterpart controls (61.5% compared with 15.4%, P < .05; Figure 7D). We also analyzed cell-cycle changes under low-dose 5-FU treatment. The MllPTD/WT LSK population showed a significant increase in S + G2/M phase, but reduction in G0/G1 phase, compared with WT controls (Figure 7E, P < .01). These results could explain the data from the CFU-spleen and competitive BMT assays. Namely, stress appears to alter the MllPTD/WT HSPCs in such a way that is permissive for rapid expansion and proliferation/survival in vivo.
Rapid expansion and reduced apoptosis of MllPTD/WT LSK/SLAM+ cells under stresses. (A) A single dose (150 mg/kg) of 5-FU was administered intraperitoneally into MllPTD/WT or WT (6-8 mice per group). BM cells were collected at the mentioned time point. Absolute number of LSK (A) and LSK/SLAM+ (B) in WT and MllPTD/WT expressed as mean ± SD per million BM cells. *P < .05. (C) Fold change of LSK or LSK/SLAM+ between day 14 and day 0 in WT and MllPTD/WT mice. (D) Apoptosis was checked by annexin V staining. Data shown are the mean percentage ± SD of annexin V+/7 AAD− and annexin V+/7 AAD+ (n = 4). *P < .05. (E) Cell-cycle analysis was performed with BrdU flow kit. Percentage of cycling cells (G0/G1 and S/G2/M) are shown for LSK fraction at 10 days after 5-FU administration (2 experiments, n = 4). **P < .01. (F) Expression of Bcl-2 family protein in LSK fractions. BM cells were harvested 24 hours after 5-FU (150 mg/kg) intraperitoneal injection (F) or collected from primary 1:1 ratio competitive BMT recipients 2 months after transplantation (G). LSK cells were selected by using autoMACS. Western blots were done using the indicated antibodies (anti-Mcl1, anti-Bcl2, anti–Bcl-XL, anti-Bax, and antitubulin).
Rapid expansion and reduced apoptosis of MllPTD/WT LSK/SLAM+ cells under stresses. (A) A single dose (150 mg/kg) of 5-FU was administered intraperitoneally into MllPTD/WT or WT (6-8 mice per group). BM cells were collected at the mentioned time point. Absolute number of LSK (A) and LSK/SLAM+ (B) in WT and MllPTD/WT expressed as mean ± SD per million BM cells. *P < .05. (C) Fold change of LSK or LSK/SLAM+ between day 14 and day 0 in WT and MllPTD/WT mice. (D) Apoptosis was checked by annexin V staining. Data shown are the mean percentage ± SD of annexin V+/7 AAD− and annexin V+/7 AAD+ (n = 4). *P < .05. (E) Cell-cycle analysis was performed with BrdU flow kit. Percentage of cycling cells (G0/G1 and S/G2/M) are shown for LSK fraction at 10 days after 5-FU administration (2 experiments, n = 4). **P < .01. (F) Expression of Bcl-2 family protein in LSK fractions. BM cells were harvested 24 hours after 5-FU (150 mg/kg) intraperitoneal injection (F) or collected from primary 1:1 ratio competitive BMT recipients 2 months after transplantation (G). LSK cells were selected by using autoMACS. Western blots were done using the indicated antibodies (anti-Mcl1, anti-Bcl2, anti–Bcl-XL, anti-Bax, and antitubulin).
To understand the divergent regulation of survival signaling in MllPTD/WT BM cells at the molecular level when under normal and stress conditions, we harvested LSK cells from 5-FU–treated mice (both MllPTD/WT and WT controls) and analyzed the expression of apoptosis-regulating proteins, specifically, Bcl2 family proteins. Among the members of Bcl2 family genes, Bfl1/A1 and Bcl-w were nearly undetectable at both RNA and protein levels (data not shown). We found down-regulation of Mcl-1 and Bcl-2 before and after 5-FU treatment in the MllPTD/WT LSK BM cells and in their counterparts (Figure 7F). Interestingly, reduced expression of both Mcl-1and Bcl-2 in MllPTD/WT cells might explain increased MllPTD/WT cell apoptosis at steady state. However, Bcl-XL was significantly up-regulated (up to 10-fold) in MllPTD/WT 5-FU-treated BM LSK cells, but not in WT LSK cells. We did not find significant changes in Bax or tubulin (Figure 7F). This might explain the increased resistance to apoptosis observed in MllPTD/WT LSKs exposed to 5-FU stress. We also measured the expression of these apoptosis-regulating proteins from the MllPTD/WT LSK BM cells and in their WT counterparts purified from BMT recipients 8 weeks after BMT. Transplantation, as similar to 5-FU treatment, induces stress on donor HSPCs. We found similar down-regulation of Mcl-1 and Bcl-2, up-regulation Bcl-XL (5-fold) in the MllPTD/WT LSK BM cells from transplanted recipient mice compared with WT LSK cells from transplanted recipient mice (Figure 7G). These data show that MllPTD/WT LSK cells have a survival advantage under multiple stress conditions compared with WT controls LSK cells.
Discussion
We examined a mouse model of Mll-PTD to understand the mechanism underlying HSC self-renewal and abnormal phenotypes associated with human MLL-PTD–positive MDS and AML. We found that murine MllPTD/WT HSPCs exhibit elevated apoptosis at steady state but become proliferative and resistant to apoptosis when expose to stress. Abnormal self-renewal activity provides the MllPTD/WT HSPCs a greater expansion advantage concurrent with both lymphoid lineage bias and a myeloid terminal differentiation blockade. Although we did not observe frank MDS or AML development in MllPTD/WT mice, their HSPC phenotypes reflect major features of human MDS. Patients with MDS display hypercellular or hypocellular marrow with dysplastic morphology and impaired maturation (dysmyelopoiesis) in the BM, and PB cytopenias. These hematopoietic defects are thought to manifest when a clonal HSC mutant predominates in the BM, suppressing healthy HSC function. In the early stages of disease, the primary cause of cytopenia is thought to be the result of reduced self-renewal, increased apoptosis, and blocked differentiation. In about one-third of patients, MDS progresses to secondary AML as additional genetic abnormalities are acquired. Little is known about the molecular mechanisms underlying MDS-associated ineffective hematopoiesis, clonal expansion, and leukemic transformation; therefore, new targeted therapies and experimental models are limited.28
A limited number of HSPCs of MllPTD/WT mice could outcompete the WT HSPCs in the same BMT assay. These results indicated that the Mll-PTD provides an advantage for clonal expansion. A second possibility is that these mutant HSC cells suppress the growth and/or survival of the WT HSCs in vivo. Our data also suggest that limiting numbers of Mll-PTD cells undergo clonal expansion over time and eventually outcompete the normal stem cells to become the dominant clone in the BM. This model probably describes the natural progression and development of an abnormal phenotype associated with MDS or AML in the presence of a hematopoietic “stressor.” To our knowledge, this is the first report supporting that the MLL-PTD defect functions as a “driver mutation” for MDS or AML stem cell expansion. However, the Mll-PTD by itself does not fully transform the aberrant HSPCs to MDS or AML.
Our previous report has shown that HoxA genes are increased in Mll-PTD mice. HoxA genes have been shown to be important for proliferation and leukemic transformation. However, it is unclear whether they also promote reprogramming or enhanced self-renewal of more differentiated cells like MPP and GMP. Hoxb4 have been shown to promote self-renewal and expansion of immature cells in vitro and in vivo. It has been reported that MLL-PTD AML have up-regulated HoxB genes. Thus, up-regulated Hoxa and/or Hoxb genes could contribute the phenotypes we described here. Further investigation is needed to provide new insight into how MLL-PTD stimulates these altered differentiation and repopulating properties.
Mutations, such as FLT3-ITD or RUNX1, cooperate with MLL-PTD for AML development.5,19 New animal models and mechanistic studies are warranted for modeling human MLL-PTD–mediated MDS and AML in mice. We found that the MllPTD/WT HSPCs, including the GMP population cells, exhibit self-renewal and repopulation activities. Thus, the MDS or leukemogenic clones do not have to be derived from LT-HSCs. This would imply that there is an increased pool of defective HSC (eg, the cancer “cell of origin”) that is intrinsically permissive to acquire additional mutations for MDS or AML development. It has recently been shown that human leukemogenesis requires multiple gene mutations.27-29 Although MllPTD/WT mice have fully mature cell types in blood, their HSPCs do not undergo normal differentiation as indicated by a strong myeloid lineage blockade in the ST-HSC, MPP, and GMP populations after BMT and in the limiting dilution BMT (Figure 4D; and data not shown). These data demonstrate that the Mll-PTD cannot only alter self-renewal of HSPCs but also induce intrinsic defects toward myeloid lineage differentiation. MLL translocations are involved in AML or ALL (both B- and T-ALL); however, MLL-PTD has been identified only in MDS and AML.5,6,31 The normal B-/T-cell differentiation we found in the MllPTD/WT HSPCs BMT assay suggests that the Mll-PTD is able to disrupt myeloid (but not lymphoid) lineage differentiation. Notably, the MllPTD/WT mice HSPC phenotypes observed in our previous reports23,24 and in this study are noticeably different from the Mll-Af9 genetic knock-in mouse model that gives rise to AML spontaneously in a short period of time.32,33 MLL-AF9, which is described as a gain-of-function mutant, is a potent oncogene for AML development. In contrast, a genetic loss-of-function model for Mll in adult hematopoiesis, MllΔ/Δ, leads to an acute BM failure, which suggests that Mll plays an important role for HSPC fitness and maintenance.34 Compared with the other 2 genetic models, the molecular mechanism underlying the unique features of MllPTD/WT mice HSPC remains to be elucidated. Future efforts to identify the downstream target genes of the Mll-PTD protein should provide mechanistic insight into these HSPC phenotypes.
We found that MllPTD/WT HSPCs are reduced in absolute number during aging, in part because of increased apoptosis. Despite reduced cell survival potential, these cell populations have a proliferative advantage in in vitro colony replating assays, in in vivo CFU-spleen assays, and rapidly expand when transplanted into recipient mice. This appears to be partly because of a Bcl-XL–mediated prosurvival pathway that is preferentially induced in donor MllPTD/WT HSPCs by the stress conditions intrinsic to transplantation. Bcl-XL has been shown play an important role for the survival and clonal expansion of HSPCs in retroviral transduction followed by BMT or retroviral random integration mediated Bcl-XL gene activation in BMT assays.35,36 Although MllPTD/WT LT-HSCs outcompete WT LT-HSCs in vivo, the MllPTD/WT-derived ST-HSCs/MPP and GMP populations have self-renewal capability, rescuing hematopoiesis by giving rise to long-term repopulating cells in recipient mice with an unexpected myeloid differentiation blockade. These findings could help explain the advantage of those HSPCs with MLL-PTD in MDS, secondary sAML, and de novo AML.
Our identification of down-regulated Mcl1 and Bcl2 might be relevant to human MDS because it has been suggested that down-regulation of MCL-1 or BCL2 can be pathogenic in MDS.37-43 Antiapoptosis therapy, such as cytokine therapies, has been suggested.44-46 However, this should be taken with additional consideration for specific patient groups, as we found up-regulation of Bcl-XL of MllPTD/WT LSK cells exposed to stress, such as 5-FU treatment or BMT. Some cytokines (eg, erythropoietin and thrombopoietin) up-regulate Bcl-XL expression.47,48 Those cytokines induce differentiation, erythropoiesis, and thrombopoiesis, which might benefit low-risk MDS but might also put high-risk patients, such as MLL-PTD+ patients, at risk for clonal expansion of abnormal HSPCs.
In conclusion, the MllPTD/WT mouse model provides unique genetic and biochemical tools to identify new targets and pathways responsible for the altered differentiation/repopulating properties, self-renewal activity, lineage bias, and myeloid differentiation blockade relevant to MLL-PTD MDS and AML. This model should also help us to understand the underlying mechanism(s) for each of the phenotypes we found in this study and facilitate improved therapies and patient outcomes in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Cincinnati Children's Hospital Research Foundation, the OCRA (G.H.), a Pelotonia Graduate Fellowship (N.Z.), the National Institutes of Health (CA89341, M.A.C.; CA140158, M.A.C. and G.M.; and CA41456, D.G.T.), and the National Natural Science Funds (81070403; Z.X.). The mouse BMT services were conducted by the Comprehensive Mouse and Cancer Core in Cancer and Blood Diseases Institute at Children's Hospital Research Foundation, which is supported through the NIDDK Centers of Excellence in Molecular Hematology (P30DK090971).
National Institutes of Health
Authorship
Contribution: Y.Z., X.Y., G.S., and G.H. designed the research; Y.Z., X.Y., G.S., X.Z., Y.R., S.G., S.P.W., N.Z., K.B., R.M.C., and G.H. performed research; Q.W., D.G.T., Z.X., G.M., J.C.M., H.L.G., and M.A.C. contributed vital new reagents; D.W., Q.W., Z.X., G.M., J.C.M., H.L.G., M.A.C., and G.H. analyzed data; and Y.Z. and G.H. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gang Huang, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Room S7.607, MLC 7013, Cincinnati, OH 45229-3039; e-mail: gang.huang@cchmc.org.
References
Author notes
Y.Z., X.Y., and G.S. contributed equally to this study.