Abstract
Introduction: The prognostic role of serum ferritin in adult patients (pts) with newly diagnosed and/or relapsed acute leukemia remains undefined. Significantly elevated serum ferritin level (>5,000 or >10,000) was a sensitive and specific for hemophagocytic lymphohistiocytosis (HLH). The outcome of adults with malignancy-associated HLH (M-HLH) is dismal (Tamamyam et al., Cancer 2016). Early diagnosis and timely therapy in these critically ill pts may be delayed while waiting for specialized labs such as IL-2Rα. We hypothesized that elevated serum ferritin might be prognostic in adult pts with acute leukemia and may serve as a rapid marker for a systemic cytokine process.
Methods: 1819 pts with AML and 260 pts with ALL evaluated at our institution between Jan 2010 to Jan 2016 with a serum ferritin level within 30-days of initial presentation were reviewed. Survival was calculated from the date of ferritin testing. Kaplan-Meier product limit method was used to estimate the median overall survival (OS) and Cox proportional hazard models were used to model the association between survival and risks factors. Recursive partitioning (RP) was used to identify optimal cut-off of ferritin levels.
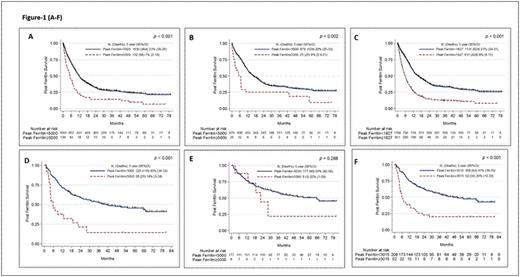
Results: In the 1819 pts with AML, median (med) age was 63 years, 57% males, the med ferritin value was 1,276 ng/ml (range, 4 - 68,262). Fifty% (n=904) pts were previously untreated. AML pts with high ferritin had significantly higher WBC count, lower platelet count, lower albumin, and high LDH (P<0.05 in each) and these remained significant when only previously untreated pts were analysed. Higher prevalence of complex karyotype, prior treatment, FLT3 and NPM1 mutations were seen in pts with high ferritin and only NPM1 remained significant when previously untreated pts were analyzed. Overall pts with AML with ferritin ≥5,000 had a significantly inferior med OS (5.1 vs 11.6 months; P<0.001) (Figure 1A). The med OS remained inferior in previously untreated pts with AML with ferritin ≥5,000 (6.6 vs 16.3 months, P=0.002) (Figure 1B). By RP analysis we found an optimal cut-off of < or ≥ 1827 ng/ml which discriminated survival in all pts with AML (Figure 1C) and < or ≥ 903 when only previously untreated pts with AML were considered. Ferritin ≥ 1827 significantly predicted for increased risk of death in MVA [HR 95% CI 1.29 (1.03-1.62) (p=0.03)] along with complex karyotype, lower hemoglobin, prior treatment, and low prevalence of CBF-AML when all AML pts were analysed. In untreated AML pts, a level of ≥ 903 predicted for increased risk of death in univariate but not in MVA.
In ALL pts (n=260), the med ferritin was 1081 ng/ml (range 20-72,060). Overall ALL pts with ferritin ≥5,000 (n=28) vs with <5000 (n=232) had significantly higher prevalence of elevated LDH, complex karyotype, and prior therapy, while in previously untreated pts with ferritin ≥5,000 (n=8 vs n=177) only elevated LDH was significant. Pts with ferritin ≥5,000 had significantly inferior med OS when all pts were included (Figure 1D), however in previously untreated pts, the difference was not significant (Figure 1E). By RP analysis, ferritin levels ≥ 3015 significantly predicted for increased risk of death in MVA [HR 95% CI 2.51 (1.57-4.02) (p<0.001)] (Figure 1F) along with prior treatment, high % of BM blasts and low albumin levels in all pts. No cut off could be identified in untreated ALL pts.
Finally we compared the outcomes of untreated AML pts with ferritin ≥5,000 (n=25) to pts with definitive M-HLH (n=15) from our institution (Tamamyam et al., Cancer 2016) and noted a similar median OS (6 and 4 months, P=0.79).
Conclusions: In this analysis, we have shown that adult pts with untreated and previously treated AML and ALL pts with ferritin ≥5,000 have an inferior survival. The optimal cut-off levels that discriminated survival were: 1807 (all AML), 903 (untreated AML), 3015 (all ALL). Untreated AML pts with ferritin ≥5,000 had a med OS of 6 months similar to pts with M-HLH. Significantly elevated ferritin in acute leukemia pts with febrile illness may be a marker to prompt a diagnostic work up for an underlying pathologic inflammation and potential benefit of augmented or targeted immune suppression.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Daver:Kiromic: Research Funding; BMS: Research Funding; Karyopharm: Honoraria, Research Funding; Ariad: Research Funding; Sunesis: Consultancy, Research Funding; Otsuka: Consultancy, Honoraria; Pfizer: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal