TO THE EDITOR:
With the increasing adoption of treatment-free remission (TFR) as a goal for patients with chronic myeloid leukemia (CML), rigorous molecular monitoring has been recommended to ensure timely tyrosine kinase inhibitor (TKI) recommencement in the event of molecular relapse. The National Comprehensive Cancer Network (NCCN) has now incorporated TFR into its most recent guidelines, recommending monthly quantitative polymerase chain reaction monitoring of BCR-ABL1 for the first 12 months following TKI discontinuation.1 Molecular relapse, the trigger to restarting TKI, is currently defined as loss of major molecular response2 (MMR; BCR-ABL1 ≤ 0.1% International Scale), and predominantly occurs in the first 6 months following TKI cessation.3,4 Delay in detection of molecular relapse may place patients at unnecessary risk of adverse outcomes, such as loss of complete hematological response. At a minimum, it delays the reachievement of MMR and deep molecular response (MR4; BCR-ABL1 ≤ 0.01% or MR4.5; BCR-ABL1 ≤ 0.0032%). Conversely, an overly rigorous monitoring schedule imposes unnecessary costs associated with a TFR attempt, adding to logistical difficulties experienced by some patients in accessing highly sensitive, standardized BCR-ABL1 testing,5 and may prevent some patients from stopping therapy. This is especially important in countries where funding of molecular testing is limited, and patients may be required to pay for additional tests.
Recommendations for qualification for a TFR attempt include a minimum of 3 years of prior TKI therapy and ≥2 years of deep molecular response.1,6 Studies have shown that the median BCR-ABL1 doubling time for patients with relapse is 9 days (range, 6.9-26.5 days)7 or ∼1 log per month.3,8 Assuming patients attempt TFR with stable MR4.5 and that the rise of BCR-ABL1 follows this linear model,7 patients who fail a TFR attempt will take at least 2 months to lose MMR. Thus, a reduction in monitoring to every 2 months should still capture the majority of patients losing MMR while still in a complete cytogenetic response (CCyR; equating to a BCR-ABL1 of <1%).9 Furthermore, as molecular relapse is most likely to occur in the first 6 months following TKI cessation,3,4 concentrating testing in this time frame will be more cost-effective. Less frequent monitoring can potentially follow.
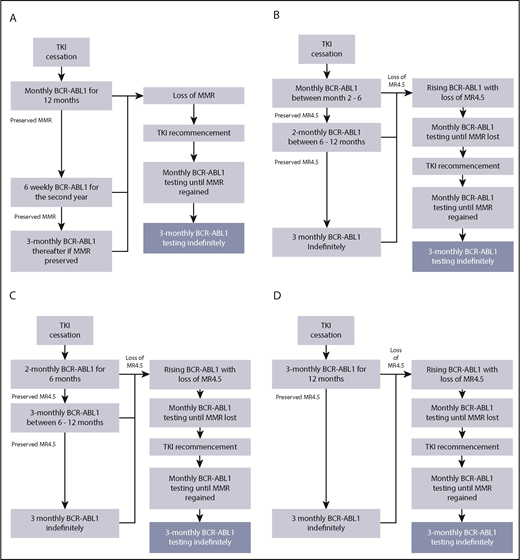
We assessed the impact of 4 monitoring algorithms (Figure 1). Figure 1A delineates the current NCCN recommendations of monthly molecular monitoring after cessation (algorithm A; 12 tests for all patients) and alternative algorithms with less frequent monitoring (algorithms B-D). All of these algorithms include an absolute requirement for monthly monitoring if there is loss of MR4.5. Algorithm B commences monthly testing at month 2 for the first 6 months, followed by monitoring every 2 months. Algorithm C introduces testing every 2 months for 6 months followed by testing every 3 months. Algorithm D uses monitoring every 3 months.
TFR monitoring based on the NCCN guidelines and potential monitoring strategies. (A) Algorithm A: based on NCCN guidelines. Monitoring regime as recommended by the NCCN, monthly testing for the first 12 months of a TFR attempt (12 tests for all patients). (B) Algorithm B commences monthly testing at month 2 for the first 6 months post-TKI cessation followed by monitoring every 2 months between 6 and 12 months. (C) Algorithm C commences testing every 2 months for the first 6 months post-TKI cessation followed by 3-monthly monitoring. (D) Algorithm D maintains 3-monthly testing throughout the TFR attempt. All algorithms incorporate monthly testing following loss of MR4.5.
TFR monitoring based on the NCCN guidelines and potential monitoring strategies. (A) Algorithm A: based on NCCN guidelines. Monitoring regime as recommended by the NCCN, monthly testing for the first 12 months of a TFR attempt (12 tests for all patients). (B) Algorithm B commences monthly testing at month 2 for the first 6 months post-TKI cessation followed by monitoring every 2 months between 6 and 12 months. (C) Algorithm C commences testing every 2 months for the first 6 months post-TKI cessation followed by 3-monthly monitoring. (D) Algorithm D maintains 3-monthly testing throughout the TFR attempt. All algorithms incorporate monthly testing following loss of MR4.5.
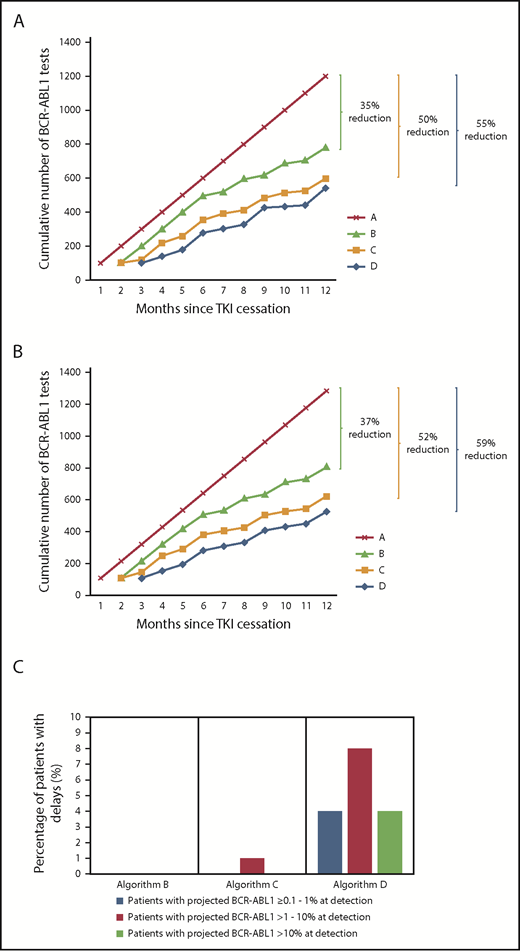
A theoretical cohort of 100 patients was used to predict the impact of reduced monitoring frequencies for patients attempting TFR, recommencing TKI in the event of MMR loss. Based on European Stop Kinase Inhibitor (EURO-SKI) data, 39% of patients will relapse and recommence TKI by 6 months with a further ∼7% predicted to lose MMR by 12 months.6 Following TKI recommencement, the NCCN recommends monthly BCR-ABL1 monitoring until MMR is regained followed by resumption of testing every 3 months.1 We estimated the number of tests over the first 12 months based on the EURO-SKI molecular relapse rate,6 the kinetics of molecular relapse3,7,8 (supplemental Methods, available on the Blood Web site), and the increased monitoring requirement following TKI recommencement according to the 4 algorithms outlined. Compared with algorithm A, each alternative algorithm decreased the total number of BCR-ABL1 tests in the theoretical cohort (Figure 2A); algorithms B, C, and D would reduce testing by 35%, 50%, and 55%, respectively.7
Cumulative frequency of BCR-ABL1 testing over the course of the first 12 months following TKI cessation using the various testing algorithms demonstrated in Figure 1. (A) Theoretical cohort of 100 patients. (B) Real cohort. One hundred seven real patients who underwent a TFR attempt and were monitored at our institution. (C) Predicted effect of detection delays based upon algorithms B-D for the 107 real patients.
Cumulative frequency of BCR-ABL1 testing over the course of the first 12 months following TKI cessation using the various testing algorithms demonstrated in Figure 1. (A) Theoretical cohort of 100 patients. (B) Real cohort. One hundred seven real patients who underwent a TFR attempt and were monitored at our institution. (C) Predicted effect of detection delays based upon algorithms B-D for the 107 real patients.
The different monitoring algorithms were then retrospectively applied to the actual data of 107 patients who attempted TFR with monitoring at our institution (supplemental Methods). The TKIs at the time of cessation were imatinib (68%; n = 73), nilotinib (21%; n = 22), dasatinib (10%; n = 11), or bosutinib (1%; n = 1). The actual number of tests per patient was 12 (total, 1284). Molecular relapse occurred in 49 patients (46%) by 12 months and 54% remained off TKI, consistent with previous studies.2-4,10-12 TKI was recommenced in 5 patients with rising BCR-ABL1 values on consecutive tests prior to MMR loss, all of whom were predicted to lose MMR within 1 to 2 months based on the calculated doubling times.7 Loss of CCyR occurred in 7 patients (7%) at the time of molecular relapse and TKI was recommenced within 1 month of molecular relapse in 38 of 49 patients. Seven patients recommenced TKI between 1 and 1.5 months following the trigger to TKI recommencement and 4 recommenced TKI 1.7 to 2.3 months following molecular relapse. One of these 4 had >10% BCR-ABL1 and complete hematological response loss, which was related to delayed TKI recommencement secondary to patient concerns regarding previous TKI toxicity. All patients rapidly regained MMR (median, 2.0 months) with no significant difference in the BCR-ABL1 halving times13 among patients who restarted TKI with BCR-ABL1 values of 0.1% to 1% vs >1%, confirming TKI sensitivity on recommencement regardless of BCR-ABL1 value.
The estimated cumulative number of BCR-ABL1 tests over 12 months after TKI cessation using actual patient data in each of the 4 monitoring algorithms is shown in Figure 2B. Three patients lost MR4.5 early, with fluctuating BCR-ABL1 levels, but remained in MMR at 12 months; such patients require monthly testing regardless of which monitoring algorithm is applied. Application of algorithm B would have resulted in a 37% reduction in testing (807 tests; average, 7.5 tests per patient). Importantly, no delay in molecular relapse detection or restarting TKI was predicted (Figure 2C). A reduction of 52% (621 tests; average, 5.8 tests per patient) would occur if algorithm C were used. One patient was predicted to have a delay of 1 month to detection of molecular relapse and restarting TKI. This would likely result in loss of CCyR at the time of relapse detection as calculated from the BCR-ABL1 doubling time.7 Monitoring patients according to algorithm D would account for the largest reduction in testing at 59% (525 tests; average, 4.9 tests per patient). However, this algorithm would delay the detection of molecular relapse by 1 to 2 months in 17 patients (16%) with associated TKI recommencement delays. Furthermore, 13 of these 17 patients were projected to lose CCyR, and 4 of the 13 were projected to have BCR-ABL1 > 10% at the time of relapse detection.
The monthly monitoring undertaken in the first TKI studies was a cautious measure instituted at a time when the potential risks of discontinuing TKI therapy were unknown. We have demonstrated that reduced frequency of monitoring in the first 12 months of a TFR attempt is likely to be safe. Planned monitoring frequency of every 2 months in the first 6 months and every 3 months between 6 and 12 months (algorithm C) may provide the best balance between reduced testing and minimization of delays in relapse detection and TKI recommencement. Patients and clinicians in settings without constraints on molecular monitoring may still prefer more frequent testing for the reassurance that it provides. We recommend re-initiation of monthly BCR-ABL1 testing at the loss of MR4.5, including in the few patients with fluctuating BCR-ABL1 levels without MMR loss, which was 3% of our cohort. We have not examined the recommendation for stringent monthly monitoring in patients requiring TKI recommencement due to molecular relapse. While resistance to TKI re-treatment is exceedingly rare, we agree with the current cautious recommendation of monthly monitoring until regaining MMR and subsequent monitoring every 3 months thereafter.1 Our data show that BCR-ABL1 can increase substantially in the time between detection of molecular relapse and the restarting of TKI therapy. This highlights the importance of a rapid turnaround time for BCR-ABL1 results (and clinical action to the result), especially with reduced monitoring frequency. Less frequent monitoring would make TFR attempts more cost-effective. More importantly, in some settings, reduced monitoring may enable clinicians to offer TFR to patients for whom the availability of molecular monitoring is a barrier. Further prospective studies are needed to validate this proposal prior to incorporation into the current standard of care.
The online version of this article contains a data supplement.
Acknowledgments
This study was conducted with local institutional review board ethical approval and as per the Declaration of Helsinki. The CML8 and CML10 studies were conducted and sponsored by the Australasian Leukaemia and Lymphoma Group, and the authors hereby thank all contributors and patients associated with these clinical studies.
N.S. received scholarship funding from the Royal Adelaide Hospital Research Foundation Dawes Scholarship. D.T.Y. is an Early Career Research Fellow of the National Health and Medical Research Council of Australia. S.B. received support from the National Health and Medical Research Council of Australia (APP1104425). T.P.H. received support from the National Health and Medical Research Council of Australia (APP1135949) and has the financial support of Cancer Council SA’s Beat Cancer Project on behalf of its donors and the State Government of South Australia through the Department of Health.
Authorship
Contribution: N.S. collected and analyzed the data and wrote the manuscript; J.A.B., A.S.M.Y., and D.K.H. reviewed the manuscript; D.T.Y. contributed data from the CML10 study and reviewed the manuscript; D.M.R. contributed data from the CML8 study and reviewed the manuscript; S.B. contributed to concept development and reviewed the manuscript; and T.P.H. conceptualized and reviewed the manuscript.
Conflict-of-interest disclosure: N.S. received honoraria from Novartis and Bristol-Myers Squibb and travel and accommodation expenses from Novartis, Gilead, Amgen, and Janssen. A.S.M.Y. received research funding from Novartis, Bristol-Myers Squibb, and Celgene and honoraria from Novartis and Bristol-Myers Squibb. D.K.H. received honoraria from Novartis. D.T.Y. received research funding from Novartis and Bristol-Myers Squibb and honoraria from Pfizer, Amgen, Novartis, and Bristol-Myers Squibb. D.M.R. received research funding from Novartis and Celgene and honoraria from Novartis, Bristol-Myers Squibb, and Celgene. S.B. is a member of the advisory boards of Qiagen, Novartis, and Bristol-Myers Squibb and received honoraria from Qiagen, Novartis, Bristol-Myers Squibb, and Cepheid. T.P.H. holds a consultancy role in, and has received research funding and honoraria from, Novartis, Bristol-Myers Squibb, and Ariad. J.A.B. declares no competing financial interests.
Correspondence: Naranie Shanmuganathan, Department of Haematology, Royal Adelaide Hospital, Port Rd, Adelaide, SA 5000, Australia; e-mail: naranie.shanmuganathan@sa.gov.au.
REFERENCES
Author notes
T.P.H. and S.B. are equal senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal