Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematologic malignancy with historically poor outcomes and no worldwide consensus treatment approach. Unique among most hematologic malignancies for its frequent cutaneous involvement, BPDCN can also invade other extramedullary compartments, including the central nervous system. Generally affecting older adults, many patients are unfit to receive intensive chemotherapy, and although hematopoietic stem cell transplantation is preferred for younger, fit individuals, not all are eligible. One recent therapeutic breakthrough is that all BPDCNs express CD123 (IL3Rα) and that this accessible surface marker can be pharmacologically targeted. The first-in-class agent for BPDCN, tagraxofusp, which targets CD123, was approved in December 2018 in the United States for patients with BPDCN aged ≥2 years. Despite favorable response rates in the frontline setting, many patients still relapse in the setting of monotherapy, and outcomes in patients with relapsed/refractory BPDCN remain dismal. Therefore, novel approaches targeting both CD123 and other targets are actively being investigated. To begin to formally address the state of the field, we formed a new collaborative initiative, the North American BPDCN Consortium (NABC). This group of experts, which includes a multidisciplinary panel of hematologists/oncologists, hematopoietic stem cell transplant physicians, pathologists, dermatologists, and pediatric oncologists, was tasked with defining the current standard of care in the field and identifying the most important research questions and future directions in BPDCN. The position findings of the NABC’s inaugural meetings are presented herein.
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) remains an aggressive, rare, and difficult-to-manage hematologic malignancy with heterogeneous clinical manifestations, including involvement of the skin, bone marrow (BM), blood, lymph nodes (LN), and central nervous system (CNS)/cerebrospinal fluid (CSF). The true incidence is difficult to know, but historical estimates suggest that BPDCN comprises only 0.44% of all hematologic malignancies, 0.7% of all cutaneous leukemias/lymphomas, and 0.04 cases per 100 000 in the United States.1-5 With improved understanding, the modern paradigm for BPDCN includes the recognition of its extreme male predominance (3:1 up to 5:1) and incidence estimates of 500 to 1000 patients per year in the United States.3,4,6,7
In addition to its rarity, the diagnosis of BPDCN has been further hampered by many nomenclature and classification changes over the past several decades, coupled with the difficulty in making a pathological diagnosis in the absence of a singular definitive test for this complex entity.7-9 BPDCN cells characteristically express CD123, CD4, CD56, CD303, TCF4, and TCL-1, whereas certain specific lineage markers such as CD14, cCD3, CD19, and MPO are not expressed.1,10-14 Genetic mutations implicated in the pathogenesis of BPDCN include inactivating tumor suppressors (ie, TP53, RB1, CDKN1B, and CDKN2A), activating oncogenes (ie, NRAS, KRAS, FLT3, RUNX2, and HES6), activated NF-κB pathway, mutated RNA spliceosomes (ie, ZRSR2 and others), immune response gene dysregulation (IFNGR, TGFB, CLEC4C, and IFNA cluster), and epigenetic dysregulation (ie, IDH1, IDH2, TET1, TET2, and ASXL1).13,15-19 The underlying biology of BPDCN has many shared but also separate unique implicated genetic mutations which distinguish it from other myeloid malignancies and may prove to be distinct targets for precision oncology and individualized therapy in the future.20
BPDCN has been historically associated with poor outcomes, with most larger series showing a complete remission (CR) rate of only 40% to 55% by acute myeloid leukemia (AML) criteria, high early death rates during multiagent chemotherapy, and dismal median overall survival (OS) rates of 8 to 16 months.3,14,21-23
There remains no consensus approach for BPDCN treatment. Globally, multiagent chemotherapy regimens are commonly used, co-opted from regimens in AML (7+3), acute lymphoblastic leukemia (ALL) (hyperfractionated cyclophosphamide, vincristine, adriamycin, and dexamethasone [HCVAD]), or lymphoma (cyclophosphamide, doxorubicin, vincristine, prednisone), modified for older patients, with varying clinical outcomes.14,24-30 Most long-term survivors are younger patients who have received conventional chemotherapy and are able to proceed to allogeneic hematopoietic stem cell transplantation (allo-HSCT) in first CR (CR1).24,31-33 However, the reality is that for many patients with BPDCN, intensive chemotherapy regimens and/or HSCT are not viable options, as the median age at diagnosis is ∼70 years.6,10,24
A notable discovery with therapeutic implications relates to CD123 (IL3Rα) overexpression in 100% of patients with BPDCN.10,12,13 CD123 is an attractive pharmacologic target given its surface location, ubiquity on BPDCN cells, and potential favorable therapeutic gradient between normal hematopoietic stem cells and the malignant stem cells of BPDCN.34-37 Ultimately, this led to the development of novel agents targeting CD123, of which the first-in-class agent was tagraxofusp (TAG, SL401), approved for patients with BPDCN aged ≥2 years in December 2018. Tagraxofusp is not only the first approved therapy for BPDCN but also the first CD123-targeting agent across all of oncology.38 Long-term follow-up of patients with BPDCN treated with frontline tagraxofusp has shown a high overall response, with many patients bridging successfully to HSCT.39 In addition, these findings led to the growth of an entire investigational field of CD123-targeting agents, including antibody-drug conjugates, chimeric antigen receptor (CAR) T-cell therapy,40 and bispecifics, among others.41 Despite this, relapses still occur in both first line and subsequent lines of therapy, and outcomes are especially poor in the relapsed/refractory (R/R) setting, with a markedly decreased OS.3,30,42-44 It is therefore of critical importance to establish standard clinical approaches and identify novel areas for research to continue to improve patient outcomes. We have, therefore, convened this group of experts from the United States, Canada, and Mexico, forming the North American BPDCN Consortium (NABC), to discuss, analyze, and present our position on the current state of the field and propose future directions.
Methods
To understand the current standards of care for BPDCN and to decide upon some of the open questions and possible future directions for the field, we created a gathering of experts, namely the NABC. We assembled a series of formal virtual meetings and follow-up communications between October 2021 and June 2022. We invited 58 expert pediatric and adult hematologist/oncologist physicians at large academic medical centers across North America. Importantly, because BPDCN encompasses multidisciplinary fields, we ensured to include experts from dermatology, dermatopathology, HSCT, and hematopathology. All members provided substantial input and actively participated in deliberations; this document represents our consensus viewpoint. There was no pharmaceutical or other sponsorship; the meetings and the final document herein solely represent the work of the authors. We had 2 primary objectives:
To describe the United States/North American standards of care for diagnosis, management, treatment options, and approaches for patients with BPDCN
To identify urgent areas of unmet medical need, representing touchstone points for future directions in research, drug development, and innovations in the BPDCN field.
Summary of NABC findings: BPDCN standards of care
After deliberations, we identified the following essential aspects for consideration in the newly diagnosed patient with BPDCN.
Diagnosis of BPDCN
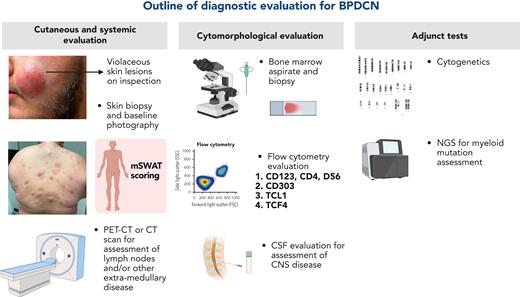
1. Uniform diagnostic approach for BPDCN. A uniform diagnostic approach for BPDCN is essential, regardless of community vs academic settings (Figure 1). The central tenet for modern diagnosis of BPDCN should include a multidisciplinary approach with the incorporation of upfront consultations with leukemia or hematology-oncology, HSCT, dermatology, and close collaboration with expert pathology (dermatopathology and hematopathology when accessible). The initial workup for each patient should include the following:
Complete blood count with differential, chemistry panel, lactate dehydrogenase, liver function tests, coagulation studies, uric acid, peripheral blood smear
BM aspiration with flow cytometry to determine phenotype
CD123 testing (flow cytometry and immunohistochemistry)
Testing for CD123, CD4, and CD56 (“Think CD123-4-56”38) and additional testing for TCL-1, TCF4,45,46 and CD30347,48 for increased specificity and to help distinguish BPDCN from other diagnoses (ie, AML or AML with leukemia cutis)
Positron emission tomography/computed tomography) or computed tomography imaging with particular emphasis on LN and extramedullary disease
LN biopsy if indicated
Dermatology consultation with full skin assessment and skin biopsy
The Modified Severity Weighted Assessment Tool can be used for objective assessment of the extent of skin involvement and can also be used for response assessment49
Lumbar puncture (LP) with intrathecal (IT) chemotherapy both for sampling of CSF (commonly positive in BPDCN) and delivery of prophylactic IT chemotherapy
Cytogenetics
Next-generation sequencing (NGS) for molecular mutations (most commonly found in BPDCN are the myeloid-centered mutations TET2, ASXL1, RAS, and TP53).13,15-18,50
Documentation of all visible skin lesions with medical photography, at baseline and at follow-up visits
Diagnostic evaluation for BPDCN. CT, computer aided tomography; mSWAT, modified severity weighted assessment tool; PET, positron emission tomography.
Diagnostic evaluation for BPDCN. CT, computer aided tomography; mSWAT, modified severity weighted assessment tool; PET, positron emission tomography.
2. Importance of distinguishing BPDCN from mimicking/similar states and the identification of prior or concomitant hematologic malignancies in BPDCN. BPDCN is now recognized as its own unique clinical entity, although it shares certain clinical similarities with other disorders, such as AML with leukemia cutis or a newer entity known as AML with expansion of plasmacytoid dendritic cells (pDC-AML).51-58 In this era of emerging targeted and individualized therapies with heterogeneous treatment responses, these unique diseases each deserve their own classification, and pDC-AML should be considered its own disease entity separate from AML. Further, roughly 10% to 20% of patients with BPDCN have also been previously or currently diagnosed with another hematologic malignancy such as ALL, myelodysplastic syndrome, chronic myelomonocytic leukemia, myeloproliferative neoplasms (MPN), or myeloma.3,6,10,59,60 It should therefore be routine practice to search for and identify any concurrent hematologic malignancies. These patients should not be excluded from BPDCN clinical trials.
Treatment approach for BPDCN
1. Systemic therapy for BPDCN as a frontline approach. We should approach BPDCN as a systemic disease and always offer systemic therapy. Importantly, there is a subset of patients with BPDCN (∼50%) that present with “skin-only” disease. Although there are differing reports, it is clear that patients with “skin-only” disease frequently experience relapse with more aggressive systemic disease and experience significant rates of treatment failure, including transformation to acute leukemia.3,14,24,26,61 Therefore, we currently view localized therapies (such as radiation) as palliative approaches to be used in very limited/specific situations.
2. Role of CD123-targeted agents. The search for a novel target for patients with AML and BPDCN led to the discovery of CD123 expression as a putative candidate.34,62 Expressed in the malignant cells of all patients with BPDCN, it represents an ideal target for drug development. Of interest, CD123 is also overexpressed in other myeloid and lymphoid hematologic malignancies.37,62 Investigation dating back to the 1970s revealed activity of diphtheria toxin either by itself or in association with immuno/lymphoid cytotoxins as antileukemia therapy.63,64 Efforts by multiple investigators found that a modified payload platform of diphtheria toxin combined with recombinant human interleukin-3, termed “DTIL3,” was found to have potent activity, both in vitro and in vivo, against BPDCN.65-68
In a phase 1 study, Frankel et al69 demonstrated major clinical responses among 7 of 9 patients, a 78% overall response rate (ORR), including 5 CRs. Remarkably, most patients received only 1 cycle of the novel agent, later known as tagraxofusp (TAG, SL401). Subsequently, Pemmaraju et al70 confirmed these results in a larger multicenter phase 1/2 study in 4 stages, which enrolled both frontline and relapsed patients. Among 29 patients treated upfront with tagraxofusp, the ORR was 90%, with a 72% rate of CR/ composite CR (CRc) (CRc indicates disappearance of all sites of disease except for residual microscopic skin abnormality); the median estimated OS was not reached in this group at a median follow-up of just over 2 years, and the OS at 2 years was 52%. The OS was not reached among frontline responding patients who received HSCT, compared with 18.3 months in patients who did not receive HSCT.70 The ORR in the R/R setting was 67%, with a median OS of 8.5 months. The major toxicity noted in trials was capillary leak syndrome (CLS), which occurred in 19% of patients, mostly grade 2 to 3, but was fatal in 2 patients during induction. Outside of this, tagraxofusp was quite manageable, with approximately half or fewer patients experiencing abnormal liver function tests, thrombocytopenia, or hypersensitivity reactions, largely limited to the first cycle of therapy. Based on this work, tagraxofusp was approved in 2018 by the US Food and Drug Administration for patients aged ≥2 years, at 12 μg/kg/d intravenous dose for 5 days per cycle. CLS was listed as a “black box warning.” Tagraxofusp was subsequently approved by the European Medicines Agency in January 2021 for first-line treatment for adults with BPDCN.38 In a longer follow-up of the above study (median of 34 months), among 64 patients in the frontline cohort who were treated at a tagraxofusp dose of 12 μg/kg, the ORR was 75%, CR/CRc was 57%, median duration of CR/CRc was around 25 months, and OS was 15.8 months; in the 19 patients with R/R disease, the ORR was 58% and median OS was 8.2 months.71
As we approach the 4-year anniversary since the approval of tagraxofusp in the United States, community adoption and administration have not revealed any new toxicity/safety signals, but providers must continue to be aware of, prevent, mitigate, and treat CLS. Long-term outcomes for tagraxofusp continue to confirm durable safety and efficacy in a subset of patients.39,72 Other agents targeting CD123, including the antibody-drug conjugate, IMGN632,73 and anti-CD123 CAR T cells74 are available in clinical trials for patients with BPDCN. IMGN632 has been given US Food and Drug Administration breakthrough therapy designation for R/R BPDCN, based on preliminary data showing an ORR of 29% (31% in patients with prior tagraxofusp), a CRc rate of 18%, and a favorable safety profile with no reported CLS occurrences.73 The IMGN632 trial is now actively recruiting patients in both frontline and R/R settings at multiple sites throughout the United States and Europe.
3. Role of venetoclax (BCL2-antagonists). The favorable clinical activity of targeting BCL-2 has been demonstrated across both lymphoid and myeloid malignancies.75 After its approval in both chronic lymphocytic leukemia as monotherapy and in AML as part of combination therapy in older adults, several groups have been investigating the role of BCL-2 and its antagonism in other leukemia subtypes.76 Montero and Lane et al investigated the role of BCL-2 and venetoclax specifically in BPDCN, finding that BPDCN is highly dependent on BCL-2 antiapoptotic function for survival and that in vitro and in vivo model systems demonstrated regression of tumor activity with administration of venetoclax monotherapy.77 Furthermore, several cases of patients with R/R disease responding to venetoclax have been described.77-80 However, the benefits of venetoclax monotherapy are often transient, suggesting a combination therapy may be preferable.81 In younger/fit patients, Pemmaraju et al30 demonstrated that combining HCVAD with venetoclax is a tolerable option in BPDCN. In older/unfit patients with BPDCN, several groups have studied a hypomethylating agent (HMA) plus venetoclax approach, similar to older AML.82-84 DiNardo et al demonstrated HMA plus venetoclax to be feasible in an off-protocol setting that included patients with BPDCN.75 Gangat et al reported that, among 10 older patients with BPDCN treated with HMA and venetoclax, 2 were bridged to HSCT.85 Active clinical trials are studying HMA plus venetoclax combinations.
4. Role for cytotoxic chemotherapy multiagent regimens. As previously discussed, several combination chemotherapy regimens adopted from other hematologic malignancies were used with variable success in BPDCN before the advent of tagraxofusp. Intensive induction cytotoxic chemotherapy regimens adapted from other hematologic malignancies (Table 1) have been associated with improved OS, especially when followed by allo-HSCT in CR1.24,41,72,86 Pemmaraju et al30 recently showed that in the modern CD123-targeted treatment era, frontline HCVAD-based multiagent regimens yield CR rates of 80%, further suggesting a continued role for chemotherapy in addition to targeted therapy in BPDCN. Further studies are needed to establish the safety profile of such combination regimens, the ideal dosing schedule, and the ability to adopt combination therapy for older/unfit patients. The choice of frontline tagraxofusp or conventional chemotherapy for patients not enrolling on a trial may be based on availability, physician/center experience, and side effect profile, as these have not been directly compared in a randomized fashion. There is no unanimous agreement nor head-to-head comparisons evaluating ALL-based vs AML-based regimens, however, most groups prefer to offer more intensive ALL-based chemotherapy as the regimen of choice for patients if they are able to tolerate treatment.27 Finally, there have been a series of preclinical data sets demonstrating that several multiple myeloma-based regimens have clinical activity in BPDCN.41,87-90
Multiagent chemotherapy regimens used in BPDCN
| Hematologic malignancy from which chemotherapy regimen was adopted . | Chemotherapy regimen . |
|---|---|
| AML | “7+3”: cytarabine, daunorubicin HiDAC: high-dose cytarabine |
| ALL | HCVAD: hyperfractionated cyclophosphamide, vincristine, adriamycin and dexamethasone ICE: ifosfamide, carboplatin, etoposide CALGB 9111: induction cyclophosphamide, daunorubicin, vincristine, prednisolone, asparaginase, and filgrastim |
| Lymphoma | CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone |
| Myeloma | Daratumumab87,88 Lenalidomide and bortezomib89,120 |
| Hematologic malignancy from which chemotherapy regimen was adopted . | Chemotherapy regimen . |
|---|---|
| AML | “7+3”: cytarabine, daunorubicin HiDAC: high-dose cytarabine |
| ALL | HCVAD: hyperfractionated cyclophosphamide, vincristine, adriamycin and dexamethasone ICE: ifosfamide, carboplatin, etoposide CALGB 9111: induction cyclophosphamide, daunorubicin, vincristine, prednisolone, asparaginase, and filgrastim |
| Lymphoma | CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone |
| Myeloma | Daratumumab87,88 Lenalidomide and bortezomib89,120 |
5. Incorporation of CNS prophylaxis as new standard of care in BPDCN. Disease involvement of the CNS has been described in up to 30% of patients with BPDCN, both in frontline and R/R settings.44,91,92 A recent report of a cohort of 103 patients with BPDCN found that 22% had CSF positivity during their disease course, with 57% of these at the time of diagnosis.44 Those with CNS involvement had a lower median baseline hemoglobin, a higher frequency of TET2 mutations, and a higher rate of BM involvement.44 An important but unknown question at this time is whether CD123-targeted agents, including tagraxofusp, IMGN632, and CAR T cells, can cross the blood-brain barrier. As patients with BPDCN go on to have longer survival with improvement in treatment options, the CNS might emerge as a sanctuary site and drive relapses in some patients. Thus, the use of CNS-directed prophylaxis is prudent as part of initial treatment.
In addition to recommending the use of IT chemotherapy as part of initial management, clinical trials and real-world analyses should evaluate the ideal timing and frequency of prophylactic screening and diagnostic LPs in patients with BPDCN.
6. Role of HSCT in CR1. Because of the efficacy and potential for cure with allo-HSCT in BPDCN,10,24,31-33 the treatment goal for all patients should be HSCT in CR1 unless otherwise contraindicated. Table 2 highlights some of the important data pertaining to response and survival outcomes of patients with BPDCN, with a focus on post-HSCT outcomes. A systematic review/meta-analysis of 4 studies showed pooled OS and disease-free survival of 67% and 53% for patients who received allo-HSCT in CR1.93 Long-term management following successful HSCT is being investigated using tagraxofusp maintenance monotherapy, with a focus on long-term remission and survival (NCT04317781). Future studies with combination therapeutics, CNS-directed therapy, and maintenance strategies should attempt to elucidate the ability to achieve cure without HSCT. In addition, autologous HSCT has been used in several series of selected patients, including those who are older/unfit for allo-HSCT or those with skin-limited disease. One series of patients with BPDCN who underwent autologous HSCT after CR1 yielded a 4-year OS of 82%, although other series have not seen as high survival after autologous HSCT.14,31,70 A North American multicenter collaborative study reported 1-year OS of only 11% in autologous HSCT recipients.94 However, both allogeneic and autologous HSCT recipients have improved OS outcomes compared with those who did not receive HSCT.72 Currently, we recommend all fit/younger patients for consideration of allo-HSCT in CR1. We did not reach consensus on the role and timing of autologous HSCT and await additional clinical research.
Overview of outcomes of patients with BDPCN from different therapy approaches with a focus on post-HSCT outcomes
| Author and study period . | Type of study . | Therapy details∗ . | Response rates [(N)/%]† . | OS dynamics . | SCT-specific outcomes . |
|---|---|---|---|---|---|
| Roos-Weil et al33 (EBMT analysis; 2000-2009) | Retrospective (only patients receiving transplant included) | Nontargeted AML/ALL-type = 27 NHL-type = 7 | N/A | Allo-SCT in CR1 = 19 Allo-SCT ≥ CR1 = 15 3 y OS = 43% | |
| Laribi et al121 2001-2017 | Retrospective | Nontargeted AML-type = 53 ALL-type = 96 NHL-type = 150 | AML-type Rx f/b SCT = 14/16 (88%) ALL-type Rx f/b SCT = 31/33 (94%) NHL-type Rx f/b SCT = 12/12 (100%) | Median OS = 18 mo | 5 years OS AML-type therapy f/b allo-SCT = 59.5% ALL-type therapy f/b allo-SCT = 47.3% NHL-type therapy f/b allo-SCT = 71.1% No allo-SCT = 9.6% for leukemia-type therapy, 8.3% for lymphoma-type therapy |
| Yun et al72 2001-2019 | Retrospective | Mixed AML-like = 1 ALL-type = 11 NHL-like = 10 SL-401 = 12 | N/A 11 (100) 9 (90) 9 (75) | mOS with SCT = NR mOS without SCT ≈ 3 y | |
| Pagano et al10 2005-2011 | Retrospective | Nontargeted AML-type = 26 ALL-type = 15 | 7/16 (44) 10/15 (67) | Median OS = 8.7 mo After AML-type Rx = 7.1 mo After ALL-type Rx = 12.3 mo | SCT = 6 mOS with SCT = 22.7 mo mOS without SCT= 7.1 mo |
| Aoki et al31 (JSHCT analysis; 2002-2015) | Retrospective (only patients receiving transplant included) | Nontargeted AML-type =4 ALL-type = 10 NHL-type = 11 | N/A | 4 y OS = 65% After AML-type Rx = 67% After ALL-type Rx = 70% After NHL-type Rx = 62% | 4 y OS After auto-SCT = 82% After allo-SCT = 69% |
| Taylor et al24 2000-2017 | Retrospective | Nontargeted AML-type = 9 ALL-type = 35 Others = 10 | N/A | 2 y OS = 49% | SCT = 25; (allo = 20, auto = 5) 2 y OS after SCT = 60% |
| Pemmaraju et al30 1999-2020 | Retrospective | Mixed ALL-type = 35 SL-401 = 37 Others = 28 | 80% 59% 43% | Median OS After ALL-type Rx= 28.3 mo After SL-401 = 13.7 mo After other Rx = 22.8 mo | % responders proceeding to allo-SCT in CR1 15/28 (54%) 13/22 (59%) 4/12 (33%) SCT specific survival data N/A |
| Pemmaraju et al71 2014-2019 | Prospective clinical trial (long-term follow-up, 34 mo) | Targeted SL-401 (F/L) = 65§ | 75% | 2 y OS = 40% | SCT = 19‡ 2 y OS after SCT = 66% 2 y OS without SCT ≈ 30% |
| Author and study period . | Type of study . | Therapy details∗ . | Response rates [(N)/%]† . | OS dynamics . | SCT-specific outcomes . |
|---|---|---|---|---|---|
| Roos-Weil et al33 (EBMT analysis; 2000-2009) | Retrospective (only patients receiving transplant included) | Nontargeted AML/ALL-type = 27 NHL-type = 7 | N/A | Allo-SCT in CR1 = 19 Allo-SCT ≥ CR1 = 15 3 y OS = 43% | |
| Laribi et al121 2001-2017 | Retrospective | Nontargeted AML-type = 53 ALL-type = 96 NHL-type = 150 | AML-type Rx f/b SCT = 14/16 (88%) ALL-type Rx f/b SCT = 31/33 (94%) NHL-type Rx f/b SCT = 12/12 (100%) | Median OS = 18 mo | 5 years OS AML-type therapy f/b allo-SCT = 59.5% ALL-type therapy f/b allo-SCT = 47.3% NHL-type therapy f/b allo-SCT = 71.1% No allo-SCT = 9.6% for leukemia-type therapy, 8.3% for lymphoma-type therapy |
| Yun et al72 2001-2019 | Retrospective | Mixed AML-like = 1 ALL-type = 11 NHL-like = 10 SL-401 = 12 | N/A 11 (100) 9 (90) 9 (75) | mOS with SCT = NR mOS without SCT ≈ 3 y | |
| Pagano et al10 2005-2011 | Retrospective | Nontargeted AML-type = 26 ALL-type = 15 | 7/16 (44) 10/15 (67) | Median OS = 8.7 mo After AML-type Rx = 7.1 mo After ALL-type Rx = 12.3 mo | SCT = 6 mOS with SCT = 22.7 mo mOS without SCT= 7.1 mo |
| Aoki et al31 (JSHCT analysis; 2002-2015) | Retrospective (only patients receiving transplant included) | Nontargeted AML-type =4 ALL-type = 10 NHL-type = 11 | N/A | 4 y OS = 65% After AML-type Rx = 67% After ALL-type Rx = 70% After NHL-type Rx = 62% | 4 y OS After auto-SCT = 82% After allo-SCT = 69% |
| Taylor et al24 2000-2017 | Retrospective | Nontargeted AML-type = 9 ALL-type = 35 Others = 10 | N/A | 2 y OS = 49% | SCT = 25; (allo = 20, auto = 5) 2 y OS after SCT = 60% |
| Pemmaraju et al30 1999-2020 | Retrospective | Mixed ALL-type = 35 SL-401 = 37 Others = 28 | 80% 59% 43% | Median OS After ALL-type Rx= 28.3 mo After SL-401 = 13.7 mo After other Rx = 22.8 mo | % responders proceeding to allo-SCT in CR1 15/28 (54%) 13/22 (59%) 4/12 (33%) SCT specific survival data N/A |
| Pemmaraju et al71 2014-2019 | Prospective clinical trial (long-term follow-up, 34 mo) | Targeted SL-401 (F/L) = 65§ | 75% | 2 y OS = 40% | SCT = 19‡ 2 y OS after SCT = 66% 2 y OS without SCT ≈ 30% |
The boldface text denotes the type of regimen used in the study mentioned in each particular row.
auto-SCT, autologous stem cell transplantation; EBMT, European society for blood and marrow transplantation; f/b, followed by; mOS, median overall survival; N/A, not available; NHL, non-Hodgkin lymphoma; NR, not reached; Rx, therapy; SL-401, tagraxofusp.
Therapy details have been broadly divided as targeted or nontargeted based on treatment with SL-401, which is targeted against CD 123.
Response rates include complete and partial remissions and annotated the best possible extent from available study data.
Includes the 19 patients who underwent SCT after CR + CRc; overall 21 patients underwent SCT.
Patients who received the 12 μg/kg dose of SL-401.
Current approach to frontline treatment in BPDCN
Younger/fit vs older/unfit patients. We recommend all patients with newly diagnosed BPDCN be referred to a large academic medical center for multidisciplinary review and enrollment in a CD123-based clinical trial approach. All patients should undergo thorough a workup, including comprehensive flow cytometric evaluation, including the markers described before for correct characterization of BPDCN, dermatological evaluation, including full-thickness skin biopsy and objective assessment of the extent of skin involvement, diagnostic LP for evaluation of occult CNS disease involvement, and further evaluation as deemed clinically prudent based on patient symptoms and clinical signs (eg, Positron emission tomography scan for extramedullary disease assessment). Younger/fit patients should be considered for upfront clinical trials, including combination therapies consisting of a CD123-targeted backbone with cytotoxic chemotherapy and/or venetoclax, all with IT chemotherapy (alternating methotrexate and cytarabine), followed by HSCT in CR1. In the community/off-protocol setting, frontline treatment options include intensive chemotherapy (favor ALL-based approach such as HCVAD ± venetoclax) or tagraxofusp monotherapy. Older/unfit patients who are not candidates for chemotherapy should be considered for clinical trials featuring CD123-targeted therapies alone or in combinations including HMA and/or venetoclax, or off-protocol “standard” approaches of tagraxofusp monotherapy or HMA plus venetoclax. In the absence of randomized trials comparing these approaches to one another, it is difficult to comment on the choice of frontline regimens in clinical practice; however, tagraxofusp monotherapy or ALL-based intensive chemotherapy in younger/fit patients and tagraxofusp monotherapy or HMA + venetoclax combination in older/unfit patients are potential choices. In 2 retrospective studies by Pemmaraju et al30 and Yun et al,72 HCVAD-based chemotherapy showed higher response rates than tagraxofusp as frontline therapy, albeit with similar OS (Table 2). In the study by Yun et al,72 there was no difference in progression-free survival among patients who received chemotherapy (HCVAD or cyclophosphamide, doxorubicin, vincristine, prednisone) or tagraxofusp when censored for allo-HSCT; however, all patients who underwent an HSCT had a significantly improved OS irrespective of the frontline therapy received (tagraxofusp or chemotherapy). However, these data have to be interpreted with caution given the disparity in the age groups, disease stages, and percentage of patients proceeding to HSCT in the different therapy arms.
The final therapy decision is thus dependent on multiple factors, including the toxicity profile of these drugs, patient and physician preference, and drug availability and affordability. As is the case for most novel targeted agents, tagraxofusp is expensive, and this can be prohibitive for some patients or centers based solely on financial considerations. The NABC acknowledged that cost may be considered in therapeutic decision-making.
Careful attention should be given to drug toxicities, supportive therapy, and patients’ quality of life (QOL) during treatment for BPDCN. As some patients might have disfiguring skin lesions on visible areas of the face and elsewhere, psychosocial issues should be considered and addressed at all times in consort with the dermatologist, supportive care team, and social worker.
Importance of clinical trials
Despite progress in the diagnosis, treatment, and survival for patients with BPDCN, there is still room for improvement. This rare disease ideally should be evaluated at a large academic center with a multidisciplinary team of experts. Furthermore, institutions should offer patients access to the latest clinical trials for treatment options, if possible. Preclinical studies are attempting to identify novel targets, such as bromodomain and extraterminal domain inhibitors as potential future options for patients with BPDCN.95,96 Phase 1 trials investigating the use of anti-CD123 CAR T-cell therapy74 are currently active for R/R BPDCN (NCT02159495 and NCT03203369). Ongoing clinical trials with triplet combination therapy regimens include tagraxofusp, venetoclax, and azacitidine (NCT03113643),84 IMGN632 plus venetoclax plus azacitidine (NCT04086264),97 as well as tagraxofusp, venetoclax, and HCVAD, plus IT chemotherapy cycles of alternating cytarabine and methotrexate (NCT04216524).38,81 Combinations may offer a “total therapy upfront approach” for BPDCN, aiming for longer durations of response, increasing upfront cure fraction, and aiming to reduce or even eliminate the need for HSCT. We believe this combination approach, incorporating all active agents in the frontline setting, represents the future of curative intent BPDCN therapy, as we have seen it applied in the AML and multiple myeloma fields.
Along with rigorously documented response, survival, and toxicity data of new treatment approaches from well-done clinical trials in BPDCN, it is also important that the feasibility of these treatment modalities be observed and studied in real-world settings. The true benefit of these therapies can be understood best when there is more community acceptance and use of these options so that the benefit might reach a wider section of patients with BPDCN.
Summary of NABC educational gaps/future research directions in BPDCN
Our panel identified 10 key areas for future direction to provide a roadmap to focus research efforts for unmet medical needs in BPDCN:
1. Augment education and awareness efforts for BPDCN as an emerging, unique entity. We recommend the continued inclusion of BPDCN in all World Health Organization editions, all malignant hematology panels/guidelines, hematology board reviews/exams, hematology/oncology fellowship curricula, along with increased efforts to publish BPDCN research in journals worldwide. Furthermore, the Consortium members aim to increase education, emphasizing the multidisciplinary nature of BPDCN clinical care and research, with efforts to reach dermatopathology, dermatology, HSCT, neuro-oncology, hematopathology, and pediatric oncology conferences, journals, and educational groups.
2. Measurable residual disease (MRD) in BPDCN. Unlike in ALL, where MRD measurement is not only standard but a crucial part of risk stratification, prognosis, and treatment decision-making, MRD testing in BPDCN is not yet standard. Our Consortium identified this area as one of the highest priority unmet medical needs in our entire field. As in AML, we must define the role of various platforms for MRD testing for BPDCN, including flow cytometry, NGS, and other modalities.98,99 At least 2 major challenges in BPDCN for MRD standardization exist currently:
Definition of MRD itself as an entity in BPDCN (how best to perform: flow cytometry vs NGS; which markers to follow).
MRD across various compartments of disease (how to uniformly characterize MRD across disease compartments and analyze differences in MRD among skin, LN, BM, and blood).
3. Aiming to eliminate HSCT in BPDCN. Once we have defined and standardized MRD specific to BPDCN, we can perhaps better establish the need for and utility of HSCT in certain patient subsets. As most patients with BPDCN are older/unfit with multiple comorbidities, progress without the need for HSCT, using combination therapies, and improved MRD testing, could represent a major breakthrough in our field.
4. Development and standardization of combination therapies in BPDCN. We hope to further our understanding of the optimal combination treatment approach for patients with BPDCN, both in the frontline and R/R settings. Ongoing clinical trials investigating combination triplet therapies using CD123-directed therapy plus venetoclax plus cytotoxic chemotherapy (for young/fit patients) or HMA (for older/unfit patients) will guide future standardization of frontline treatment approaches. In addition, given the increasing understanding of the high rates of occult CNS disease even at the time of diagnosis, we propose to perform diagnostic LPs on all patients, as well as prophylactic IT chemotherapy as an integral part of all combination frontline treatment. Many of us follow the adult ALL paradigm for the number/schedule of LPs and IT chemotherapy delivery, regardless of therapy or clinical trial selection. It is therefore imperative that we strive to include the administration of prophylactic LPs with IT chemotherapy and allow for the treatment of active CSF+/CNS+ disease during BPDCN clinical trials.
5. Identification of the underlying/unifying disease pathobiology/pathway of BPDCN. The examples provided by the committee for a similar disease model included that of acute promyelocytic leukemia100,101 and hairy cell leukemia,102-104 both of which are also rare leukemia subtypes that have experienced the discovery of a singular, consistent aberrant pathobiological process that not only can be easily diagnosed but also can be effectively treated with curative intent using standard, universally accepted, and available targeted therapies. The quest for something similar for BPDCN is an active area of investigation, moving beyond CD123 to identify molecular pathways with single-cell sequencing, examining immune-modulatory pathways, and pDC-specific pathobiology for the next generation of BPDCN research. Emerging research areas will require improved research sample banking and sharing among investigators and will include investigation of clonal hematopoiesis and clonal evolution in BPDCN105 and a greater understanding of Toll-like receptors, cytokines, and other important immunomodulatory pathways that are becoming recognized as important in BPDCN disease biology.18,50,61,106,107
6. Pediatric BPDCN. One of our most pressing concerns was to distinguish patient characteristics, molecular differences, and management approaches between pediatric and adult BPDCN, as has been done in AML.108,109 Furthermore, the lack of pediatric-focused BPDCN clinical trials and barriers to the inclusion of pediatric patients in trials generally were noted as an urgent area in need of improvement for drug development. Notably, the tagraxofusp approval for BPDCN did include patients aged ≥2 years, but only on basis of a small retrospective cohort of treated patients, which highlights pediatric BPDCN as a rare entity within an already rare entity.110,111 Several coauthors of this expert panel are specialists in pediatric leukemia, including BPDCN; separate guidelines and principles of care will be developed in the future once more is understood for this entity.33,110,112-114
7. QOL measurement in BPDCN. Several panel members have expertise in patient-reported outcomes (PRO) and QOL measurement in acute and chronic leukemias, including MPN, and in geriatric patients with cancer.115-118 We have observed that with treatment, many BPDCN patients have experienced often immediate improvement in their constitutional symptoms, bone pain, pain from skin lesions, and psychological improvement from the disappearance of skin disfigurement. We recommend capturing these important patient-centered metrics by modeling existing questionnaires in AML and MPN and ultimately by creating and validating a BPDCN PRO/QOL questionnaire. This may be similar to Mesa et al’s116 MPN TSS, which has now been incorporated into most MPN clinical trials. Furthermore, for BPDCN, studying the correlation of disease response with PRO/QOL improvement and cytokine levels or other molecular features is of importance.
8. Development of risk stratification and prognostic markers in BPDCN. One unique aspect of BPDCN is the frequent extramedullary involvement with heterogeneous presentations at baseline and relapse. A successful risk stratification scale would ideally include molecular, cytogenetic, and any other dermatopathology or hematopathology markers/factors that could carry independent prognostic weight in a multivariate analysis.
9. Increase clinical trial access for patients with BPDCN. We recommend that all patients with BPDCN be offered clinical trial participation, including in frontline and relapsed settings. However, multiple barriers were identified:
Many patients with BPDCN are older/frail and cannot travel far distances from home for clinical trials, which often require 1 to 4-week travel commitments or more
The COVID-19 pandemic only worsened logistical/transportation/costs
Lack of central institutional review boards across sites
Increased costs of research, including conducting and monitoring clinical trials
Clinical entry criteria that rule out ill patients, those with poor performance status, or those with CNS disease
Lack of inclusion of pediatric patients
Difficulty enrolling in clinical trials outside the United States or difficulty/cost and other barriers in coming to the United States for a clinical trial from Mexico or Canada.119
The NABC calls for immediate action with the creation of a formal National BPDCN Clinical Trials Network and the inclusion of all underserved or underrepresented groups. Our goal in this deadly rare blood cancer is to offer 100% of our patients’ enrollment in clinical trials, ideally as close to home as possible. We can achieve this with increased collaboration across North America; the creation of this group of experts, we believe, is a good first step. In addition, we suggest the development of a central/national BPDCN tumor registry, or adding BPDCN to an established registry (eg, there is an active cutaneous lymphoma registry created by our dermatology colleagues). Moreover, we call for the creation of a centralized, non–pharma-sponsored Internet website that can serve as a headquarters for all patients searching for information on BPDCN. Furthermore, we call on the organization of a national/international BPDCN tumor board, in which providers for patients with BPDCN, particularly those being treated outside of experienced sites, can access expertise from our combined group.
10. Identification of foundations, grant mechanisms, philanthropy, and sources of funding for BPDCN. We note, as with any rare disease, it is an ongoing challenge to raise awareness, continue educational efforts, maintain relevancy in a competitive funding environment. In addition, despite advancements in therapy in BPDCN, the costs of targeted regimens may become a deterrent for wider community use of these drugs. Formal cost analysis of different regimens used in BPDCN is very important and remains an important goal for the consortium.
We call for greater emphasis on rare diseases and rare blood cancers in general, and advocate for specific grant opportunities to support research for BPDCN. The NABC concluded with a reminder that discoveries in the rarest disease states, oftentimes rare blood cancers, have subsequently led to breakthroughs in other, more common entities, thereby ultimately helping even more patients over time.
Acknowledgment
Figure 1 was made on Biorender.com.
Authorship
Contribution: N.P., H.K., and A.A.L. designed the manuscript; N.P., H.K., A.A.L., J.S., and N.R.W. wrote the manuscript; and all authors reviewed and analyzed the data, reviewed, edited, and approved the final manuscript.
Conflict-of-interest disclosure: N.P. has a leadership role in ASH and ASCO; received honoraria from Incyte, Novartis, LFB Biotechnologies, Stemline Therapeutics, Celgene, AbbVie, MustangBio, Roche Molecular Diagnostics, Blueprint Medicines, DAVA Pharmaceuticals, Springer, Aptitude Health, NeoPharm, and CareDX; has a consulting or advisory role in Blueprint Medicines, Pacylex, Immunogen, Bristol Myers Squibb, ClearView Healthcare Partners, Astellas Pharma, Protagonist Therapeutics, Triptych Health Partners, and CTI BioPharma Corp; received research funding from Novartis, Stemline Therapeutics, Samus Therapeutics, AbbVie, Cellectis, Affymetrix/Thermo Fisher Scientific, Daiichi Sankyo, Plexxikon, and MustangBio; received travel, accommodation, and other expenses from Stemline Therapeutics, Celgene, AbbVie, DAVA Oncology, and MustangBio; and has uncompensated relationships with Dan's House of Hope and Oncology Times. H.K. received research grants from AbbVie, Agios, Amgen, Ariad, Astex, BMS, Cyclacel, Daiichi Sankyo, Immunogen, Jazz Pharma, Novartis, Pfizer, Actinium, and Takeda. A.E.F has patent and received royalties on SL-401. S.S is in a consulting role in AbbVie, Genentech, BerGen Bio, Syros, Kura Oncology, Senti Biosciences, and Novartis. N.D received research funding from Bristol Myers Squibb, Pfizer, Immunogen, Genentech, AbbVie, Astellas Pharma, Servier, Daiichi Sankyo, Gilead Sciences, Amgen, Trillium Therapeutics, Hanmi, Trovagene, FATE Therapeutics, Novimmune, GlycoMimetics and is in a consulting or advisory role in Celgene, Agios, Jazz Pharmaceuticals, Pfizer, AbbVie, Astellas Pharma, Daiichi Sankyo, Novartis, Bristol Myers Squibb, Amgen, Immunogen, Genentech, Servier, Syndax, Trillium Therapeutics, Gilead Sciences, Arog, Shattuck Labs. M.S.T. received research funding from Abbvie, Orsenix, Biosight, Glychas omimetics, Rafael Pharmaceuticals, Amgen; has an advisor role in Abbvie, Daiichi-Sankyo, Orsenix, KAHR, Oncolyze, Jazz Pharmaceuticals, Roche, Biosight, Novartis, Innate Pharmaceuticals, Kura, Syros Pharmaceuticals, Ipsen Biopharmaceuticals, Cellularity and received roalties from UpToDate. A.A.L. received research funding from AbbVie and Stemline Therapeutics; and received consulting fees from N-of-One and Qiagen. The remaining authors declare no competing financial interests.
Correspondence: Naveen Pemmaraju, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: npemmaraju@mdanderson.org; and Andrew Lane, Department of Medical Oncology, Dana-Farber Cancer Institute, 450 Brookline Ave, Boston, MA, 02215; e-mail: andrew_lane@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal