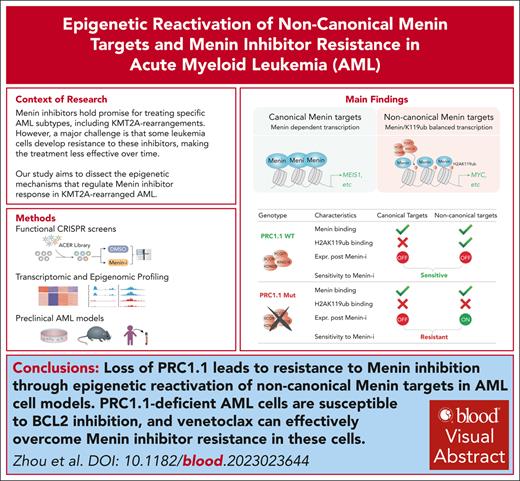
Key Points
Loss of PRC1.1 leads to resistance to menin inhibition through epigenetic reactivation of noncanonical menin targets in AML cell models.
PRC1.1-deficient AML cells are susceptible to BCL2 inhibition and venetoclax can overcome menin inhibitor resistance in these cells.
Visual Abstract
Menin inhibitors that disrupt the menin-MLL interaction hold promise for treating specific acute myeloid leukemia (AML) subtypes, including those with KMT2A rearrangements (KMT2A-r), yet resistance remains a challenge. Here, through systematic chromatin-focused CRISPR screens, along with genetic, epigenetic, and pharmacologic studies in a variety of human and mouse KMT2A-r AML models, we uncovered a potential resistance mechanism independent of canonical menin-MLL targets. We show that a group of noncanonical menin targets, which are bivalently cooccupied by active menin and repressive H2AK119ub marks, are typically downregulated after menin inhibition. Loss of polycomb repressive complex 1.1 (PRC1.1) subunits, such as polycomb group ring finger 1 (PCGF1) or BCL6 corepressor (BCOR), leads to menin inhibitor resistance by epigenetic reactivation of these noncanonical targets, including MYC. Genetic and pharmacological inhibition of MYC can resensitize PRC1.1-deficient leukemia cells to menin inhibition. Moreover, we demonstrate that leukemia cells with the loss of PRC1.1 subunits exhibit reduced monocytic gene signatures and are susceptible to BCL2 inhibition, and that combinational treatment with venetoclax overcomes the resistance to menin inhibition in PRC1.1-deficient leukemia cells. These findings highlight the important roles of PRC1.1 and its regulated noncanonical menin targets in modulating the menin inhibitor response and provide potential strategies to treat leukemia with compromised PRC1.1 function.
Introduction
Acute myeloid leukemia (AML) is a hematologic malignancy characterized by the rapid and uncontrolled proliferation of immature myeloid cells. KMT2A-rearranged (KMT2A-r) AML is an aggressive subtype of AML, constituting 5% to 10% of adult AML cases and ∼70% of childhood AML cases, with patients facing high relapse rates and poor overall survival outcomes.1,2 The KMT2A fusion proteins aberrantly recruit transcriptional machinery and chromatin remodeling complexes, activating oncogenic transcription factors, including MEIS1, PBX3, MEF2C, and the HOXA cluster genes, which collectively contribute to leukemia induction and maintenance.1
Menin is a chromatin adapter protein that directly interacts with the KMT2A protein and mediates epigenetic regulation of KMT2A target genes. The menin-KMT2A interaction has been shown to play a crucial role in promoting leukemogenesis in KMT2A-r AML, making it an attractive therapeutic target.3,4 Consequently, the development of small-molecule inhibitors that disrupt the menin-MLL1 protein-protein interaction has become a major focus in leukemia research.5-7 Several menin inhibitors are currently being evaluated in clinical trials and have shown promising early results. For example, in an ongoing phase 1/2, patients with KMT2A-r leukemia exhibited an overall response rate of 59%, with approximately one-third of the treated patients achieving complete remission or complete remission with partial hematologic recovery.8
Despite the encouraging outcomes of menin inhibitors, the mechanisms underlying therapeutic resistance remain to be explored. A recent study revealed that nearly 40% of resistance cases were attributed to mutations in the MEN1 gene that occurred during treatment.9 Although this finding is crucial, it leaves >60% of resistance unexplained, highlighting the presence of nongenetic mechanisms regulating the therapeutic response. To date, only a few nongenetic factors have been identified that regulate the menin inhibitor response, including the epigenetic modifier KDM6A, as reported by us and others.10,11 Interestingly, the primary function of menin is linked to the transcriptional regulation of downstream targets, such as MEIS1, PBX3, and MEF2C. Yet, in nongenetic resistance cases, these canonical menin targets often continue to be repressed by menin inhibitors, as seen in AML primary samples and patient-derived xenografts.8,12 This observation prompted us to hypothesize that alternative pathways might circumvent the on-target effects of menin inhibitors, leading to reduced treatment efficacy.
In this study, we conducted an integrated study combining CRISPR screens, genetic, epigenetic, and pharmacological techniques to investigate the factors influencing the response to menin inhibitors in KMT2A-r AML.
Methods
Additional details are provided in the supplemental Methods, available on the Blood website.
Advanced Catalogue of Epigenetic Regulators (ACER) library construction and CRISPR screen
Single-guide RNA (sgRNA) oligos (GenScript) were cloned into the LRG2.1_Neo (Addgene, 125593) vector using Gibson Assembly. Lentiviruses of the CRISPR library were packaged in HEK293FT cells and used to infect OCI-AML2 or MOLM-13 cells, followed by treatment with either dimethyl sulfoxide (DMSO), VTP50469, or venetoclax (as detailed in supplemental Methods). Screen libraries were constructed using Nextera primers as described before,13 followed by deep sequencing on the NovaSeq 6000 platform. sgRNA counts were mapped and analyzed using the Model-based Analysis of Genome-wide CRISPR-Cas9 Knockout (MAGeCK) method.14
Compound treatment
VTP50469 (HY-114162), venetoclax (HY-15531), ABBV-075 (HY-100015), and APTO-253 (HY-16291) were obtained from MedChemExpress. Leukemia cells were seeded in 24-well plates at a density of 2 × 104 cells per mL for the in vitro compound treatment. The treated cells were replated into new 24-well plates every 3 days with fresh medium and compound.
Animal experiments
All animal experiments were conducted with the approval of the University of Alabama at Birmingham Animal Resources Program. Animals (detailed in supplemental Methods) were randomly assigned to treatment groups and treated with vehicle, VTP50469, or venetoclax after transplantation.
Statistics
Data are presented as mean ± standard deviation from 3 independent experiments unless otherwise noted. Statistical analyses were performed using a 2-tail Student test for comparing 2 data sets with assumed normal distribution unless otherwise noted. Two-way analysis of variance was performed for comparing growth curves over time. A Fisher's exact test was performed for categorical variables. Survival analyses were performed using the log-rank test with GraphPad Prism software (v9). ∗, ∗∗, ∗∗∗, and ∗∗∗∗ denote P values <.05, .01, .001, and .0001, respectively. n.s. denotes not significant.
Results
CRISPR screen of ACER library identifies epigenetic regulators of menin inhibitor response in KMT2A-r AML
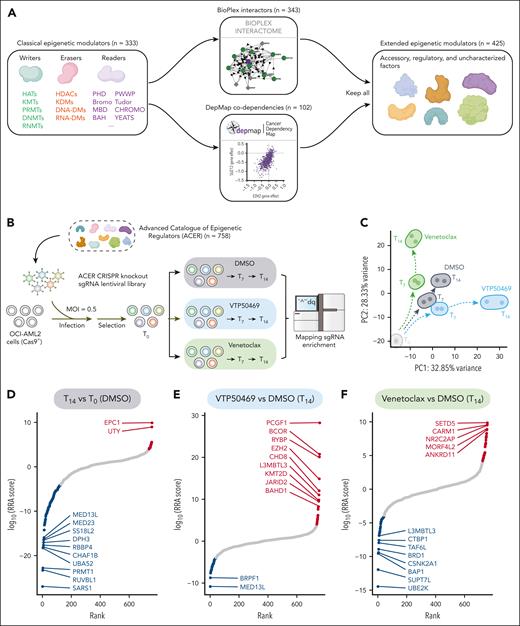
Recent advances in protein interaction and gene-dependency mapping have enabled the exploration of functionally related genes of particular interest.15,16 Here, we built a specialized collection of chromatin regulators termed ACER. First, we curated a selection of 333 well-recognized epigenetic regulators based on their established roles in chromatin modification and the presence of conserved chromatin domains (supplemental Figure 1A). We then broadened this list by integrative network analyses using gene codependency information from the Dependency Map (DepMap) database and protein-protein interaction data from the BioPlex database, resulting in a final list of 758 epigenetic regulators (Figure 1A). Gene ontology analysis supported the relevance of our collection, revealing significant enrichment in chromatin regulation pathways for both canonical and extended genes (supplemental Figure 1A). We next constructed a CRISPR knockout screening library focused on the ACER genes with 10 sgRNAs for each gene, along with 625 sgRNAs that target negative control genes (supplemental Table 1). The complexity and uniformity of the guide sequences in the ACER library were confirmed using next-generation sequencing (supplemental Figure 1B-C).
CRISPR screen of the ACER library identifies crucial epigenetic regulators of menin inhibitor response. (A) Schematic of ACER library design. Classical epigenetic modulators (n = 333) were extended by including 343 interactors from BioPlex Interactome and 102 codependency genes from the DepMap data set, resulting in a collection of 758 epigenetic regulator genes. (B) Schematic overview of the CRISPR knockout screen using the ACER library. OCI-AML2 AML cells with stable Cas9 expression were transduced with pooled sgRNAs using lentiviruses. After antibiotic selection, these cells were treated with DMSO, 1 μM VTP50469, or 100 nM venetoclax for 0, 7, and 14 days. After treatment, the genomic DNA was harvested for library construction and analyzed using next-generation sequencing. (C) Principal component analysis showing time-dependent yet distinct cellular responses of the DMSO, VTP50469, and venetoclax treatment groups. (D-F) MAGeCK analysis results showing a ranking of genes based on their RRA scores. These rankings compare the day 14 DMSO-treated group with the day 0 DMSO-treated group (D), the day 14 VTP50469-treated group with the day 14 DMSO-treated group (E), and the day 14 venetoclax-treated group with the day 14 DMSO-treated group (F). Screen hits that were significantly dropped and enriched (RRA < 0.0001) are marked in blue and red, respectively, with the gene names of the top hits labeled for reference. BAH, bromo-adjacent homology; Bromo, bromodomain; Cas9, CRISPR-associated protein 9; CHROMO, chromatin organization modifier; DNA-DMs, DNA demethylases; DNMTs, DNA methyltransferases; HATs, histone acetyltransferases; HDACs, histone deacetylases; KMTs, lysine methyltransferases; KDMs, lysine demethylases; MBD, methyl-CpG binding domain; MOI, multiplicity of infection; PC, polycomb; PHD, plant homeodomain; PRMTs, protein arginine methyltransferases; PWWP, proline-tryptophan-tryptophan-proline domain; RNA-DMs, RNA demethylases; RNMTs, RNA methylrtansferases; RRA, robust rank aggregation.
CRISPR screen of the ACER library identifies crucial epigenetic regulators of menin inhibitor response. (A) Schematic of ACER library design. Classical epigenetic modulators (n = 333) were extended by including 343 interactors from BioPlex Interactome and 102 codependency genes from the DepMap data set, resulting in a collection of 758 epigenetic regulator genes. (B) Schematic overview of the CRISPR knockout screen using the ACER library. OCI-AML2 AML cells with stable Cas9 expression were transduced with pooled sgRNAs using lentiviruses. After antibiotic selection, these cells were treated with DMSO, 1 μM VTP50469, or 100 nM venetoclax for 0, 7, and 14 days. After treatment, the genomic DNA was harvested for library construction and analyzed using next-generation sequencing. (C) Principal component analysis showing time-dependent yet distinct cellular responses of the DMSO, VTP50469, and venetoclax treatment groups. (D-F) MAGeCK analysis results showing a ranking of genes based on their RRA scores. These rankings compare the day 14 DMSO-treated group with the day 0 DMSO-treated group (D), the day 14 VTP50469-treated group with the day 14 DMSO-treated group (E), and the day 14 venetoclax-treated group with the day 14 DMSO-treated group (F). Screen hits that were significantly dropped and enriched (RRA < 0.0001) are marked in blue and red, respectively, with the gene names of the top hits labeled for reference. BAH, bromo-adjacent homology; Bromo, bromodomain; Cas9, CRISPR-associated protein 9; CHROMO, chromatin organization modifier; DNA-DMs, DNA demethylases; DNMTs, DNA methyltransferases; HATs, histone acetyltransferases; HDACs, histone deacetylases; KMTs, lysine methyltransferases; KDMs, lysine demethylases; MBD, methyl-CpG binding domain; MOI, multiplicity of infection; PC, polycomb; PHD, plant homeodomain; PRMTs, protein arginine methyltransferases; PWWP, proline-tryptophan-tryptophan-proline domain; RNA-DMs, RNA demethylases; RNMTs, RNA methylrtansferases; RRA, robust rank aggregation.
To identify chromatin regulators controlling the menin inhibitor response, we used our ACER library in a CRISPR knockout screen with OCI-AML2 cells, representative of KMT2A-r leukemia with MLL-AF6 rearrangement. The ACER library was introduced into these cells, which were then exposed to the menin inhibitor VTP50469 or treatment controls (DMSO or BCL2 inhibitor venetoclax). Guide RNA distribution was measured via deep sequencing on days 0, 7, and 14 after treatment (Figure 1B). The distribution of normalized counts over time exhibited greater changes, indicating a time-dependent response to treatment (supplemental Figure 1D). Principal component analysis also demonstrated distinct cellular responses to each treatment over time (Figure 1C). Our screen uncovered numerous epigenetic factors that control the therapeutic response to menin inhibition or BCL2 blockade, as well as the overall fitness of leukemia cells. Known regulators with anticipated roles have been successfully identified. These include genes essential for cell fitness, such as PRMT1,17 genes governing venetoclax sensitivity, such as UBE2K,18 and genes associated with resistance to menin inhibitors, including KDM6A and KMT2D10,11 (Figure 1D-F; supplemental Figure 1E-G; supplemental Table 2). Importantly, our screen has pinpointed genes associated with the Polycomb repressive complex 1 (PRC1; PCGF1, BCOR, and RYBP) and the PRC2 (EZH2 and EED) as top candidates conferring resistance to menin inhibitor treatment when depleted (Figure 1E; supplemental Figure 1F).
Loss of PRC1.1 or PRC2.2 renders menin-MLL inhibitor resistance in KMT2A-r leukemia cells
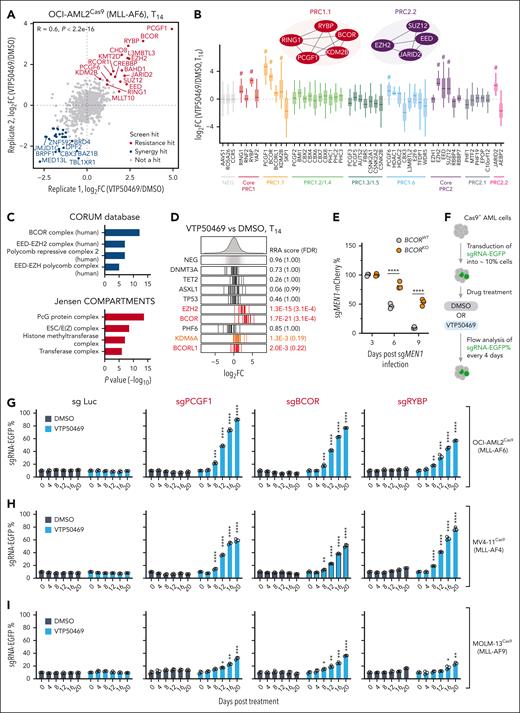
The Polycomb group (PcG) proteins in PRC1 and PRC2 form numerous canonical and noncanonical complex variants.19 We further investigated our screen results to determine if any specific PcG complex variant was involved. Our menin inhibitor screen identified 66 genes as synergistic hits and 47 genes as resistant hits, each with a robust rank aggregation score <0.001 (supplemental Table 2). Among them, we identified 25 synergistic hits, such as BRPF1, BRD4, MED13L, and JMJD1C, and 17 resistant hits including the core PRC1 complex (RING1 and RYBP), PRC 1.1 complex (BCOR, KDM2B, and PCGF1), core PRC2 complex (EED, SUZ12, and EZH2), and PRC2.2 complex (JARID2), as top reliable hits (fold change >2 and false discovery rate <0.01; Figure 2A-B). Consistently, we identified PRCs as the most significantly enriched complexes in resistance hits using the Comprehensive Resource of Mammalian Protein Complexes (CORUM) and Jensen COMPARTMENTS databases (Figure 2C). Other PcG complex variants specific to PRC1.2 to 1.6 or PRC2.1 were not enriched as resistance hits, suggesting a unique role for PRC1.1 and PRC2.2 in modulating the cell response to menin inhibition (Figure 2B).
Loss of PRC1.1 or PRC2.2 renders menin-MLL inhibitor resistance in KMT2A-r leukemia cells. (A) Scatterplot of gene log2 FC (VTP50469/DMSO) in 2 biological replicates of screens in OCI-AML2 (MLL-AF6) cells. (B) Boxplots of sgRNA log2 FC (VTP50469/DMSO) for each indicated gene in OCI-AML2 cells. # denotes resistance hits identified in the screen. (C) Enrichment analysis of resistance hit genes using the Comprehensive Resource of Mammalian Protein Complexes (CORUM) protein complex database (top) and Jensen COMPARTMENTS (bottom). (D) Bar plots showing sgRNA log2 FCs in genes with recurrent mutations in AML. The bars represent the log2 FC for each sgRNA. The density plots above represent the distribution of all sgRNAs. (E) Competitive growth assay of mCherry-tagged sgMEN1 cells in BCOR wild-type and knockout OCI-AML2 cells. The graph shows the relative percentages of sgMEN1-expressing cells at the indicated time points after sgRNA infection. The mCherry percentage at each time point was normalized to the day 3 measurement. (F) Schematic illustrating competitive growth assays to assess growth advantage of leukemia cells expressing specific sgRNA sequences, under conditions of either DMSO or VTP50469 treatment. (G-I) Competitive proliferation assays showing the relative percentage of leukemia cells expressing EGFP-tagged sgRNA sequences under either DMSO or VTP50469 treatment in the OCI-AML2 (G; 100 nM), MV4-11 (H; 30 nM), and MOLM-13 (I; 10 nM) KMT2A-r AML cell lines. sgRNA targeting the luciferase gene sgLuc was used as a negative control. The EGFP percentage at each time point was normalized to the initial measurement. EGFP, enhanced green fluorescent protein; FC, fold change; NEG, negative control; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P <.0001.
Loss of PRC1.1 or PRC2.2 renders menin-MLL inhibitor resistance in KMT2A-r leukemia cells. (A) Scatterplot of gene log2 FC (VTP50469/DMSO) in 2 biological replicates of screens in OCI-AML2 (MLL-AF6) cells. (B) Boxplots of sgRNA log2 FC (VTP50469/DMSO) for each indicated gene in OCI-AML2 cells. # denotes resistance hits identified in the screen. (C) Enrichment analysis of resistance hit genes using the Comprehensive Resource of Mammalian Protein Complexes (CORUM) protein complex database (top) and Jensen COMPARTMENTS (bottom). (D) Bar plots showing sgRNA log2 FCs in genes with recurrent mutations in AML. The bars represent the log2 FC for each sgRNA. The density plots above represent the distribution of all sgRNAs. (E) Competitive growth assay of mCherry-tagged sgMEN1 cells in BCOR wild-type and knockout OCI-AML2 cells. The graph shows the relative percentages of sgMEN1-expressing cells at the indicated time points after sgRNA infection. The mCherry percentage at each time point was normalized to the day 3 measurement. (F) Schematic illustrating competitive growth assays to assess growth advantage of leukemia cells expressing specific sgRNA sequences, under conditions of either DMSO or VTP50469 treatment. (G-I) Competitive proliferation assays showing the relative percentage of leukemia cells expressing EGFP-tagged sgRNA sequences under either DMSO or VTP50469 treatment in the OCI-AML2 (G; 100 nM), MV4-11 (H; 30 nM), and MOLM-13 (I; 10 nM) KMT2A-r AML cell lines. sgRNA targeting the luciferase gene sgLuc was used as a negative control. The EGFP percentage at each time point was normalized to the initial measurement. EGFP, enhanced green fluorescent protein; FC, fold change; NEG, negative control; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P <.0001.
A recent study indicated that the accumulation of AML mutations may result in the development of clones that are resistant to menin inhibitor therapy.12 We evaluated the impact of AML-related mutation genes included in our ACER library. Although the loss of many commonly mutated genes in AML, such as DNMT3A, TET2, ASXL1, and TP53, did not directly lead to resistance, a few specific genes, such as BCOR, BCORL1, and EZH2, along with the previously identified KDM6A, emerged as resistance hits (Figure 2D). We also observed different effects of PRC1.1 and PRC2.2 on AML cell fitness. The enrichment of sgRNAs targeting the PRC2 genes EED and SUZ12 dropped during AML cell culture under untreated conditions (supplemental Figure 2A-B), which is in line with the previously established role of PRC2 in maintaining KMT2A-r AML.20,21 Conversely, loss of PRC1.1 subunits such as PCGF1, BCOR, and RYBP did not affect AML growth and led to robust sgRNA enrichment under menin inhibitor treatment (supplemental Figure 2A-B). To further validate our screen results, we first confirmed the resistance of PRC1.1 deficient cells to menin protein depletion (Figure 2E). Next, we used a competition-based growth assay using sgRNAs targeting PCGF1, BCOR, and RYBP in response to a menin inhibitor in 3 representative KMT2A-r AML cell lines: OCI-AML2 (harboring MLL-AF6), MV4-11 (harboring MLL-AF4), and MOLM-13 (harboring MLL-AF9) (Figure 2F). In all cell models, we observed a robust competitive advantage of cells carrying sgRNAs targeting PRC1.1 and PRC2.2 complex subunits under menin inhibitor treatment (Figure 2G-I; supplemental Figure 2C-G).
PRC1.1 is required for menin-MLL inhibitor-mediated antileukemia effects
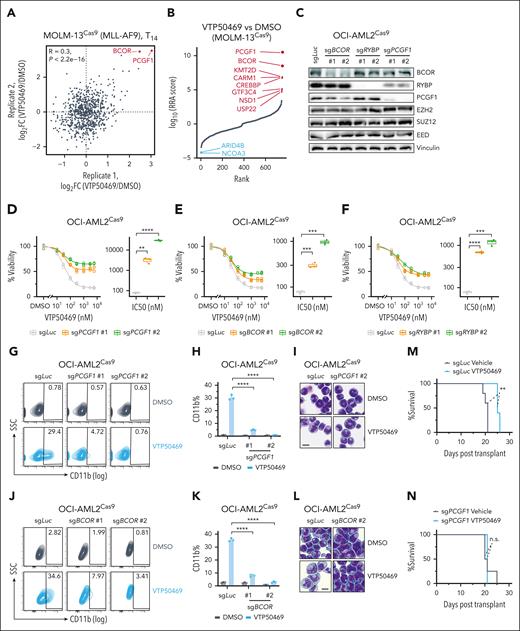
To further confirm our screen findings, we carried out an ACER CRISPR screen in MOLM-13 cells to investigate whether other potential factors contribute to menin resistance (supplemental Figure 3A). We found that PRC1.1 complex genes, particularly PCGF1 and BCOR, remained the top 2 resistant hits (Figure 3A-B), highlighting the crucial role of PRC1.1 in modulating the response to menin inhibitors across various KMT2A-r subtypes. Next, we generated stable knockout lines for PRC1.1 subunits in 3 independent KMT2A-r cell lines, which were confirmed by western blotting (Figure 3C; supplemental Figure 3B-C). It is worth noting that knocking out certain PRC1.1 members reduced the protein levels of other subunits, likely due to the destabilization of the complex. Importantly, loss of PRC1.1 conferred significant resistance to menin inhibition as measured by the cell viability assay (Figure 3D-F; supplemental Figure 3D-I). Menin inhibition is known to promote myeloid differentiation in AML cells.7 Indeed, our results showed that VTP50469 increased myeloid differentiation by measuring the myeloid marker CD11b expression and Wright-Giemsa staining. However, this effect was completely negated with the depletion of PRC1.1 components PCGF1 or BCOR (Figure 3G-L; supplemental Figure 3J-O). Resistance to menin inhibition was further evidenced by an increased leukemia burden in PCGF1-knockout MV4-11 xenograft models in vivo (Figure 3M-N; supplemental Figure 3P-O). Collectively, these results demonstrated that PRC1.1 is critically required for the antileukemic effects induced by menin-MLL inhibitors.
PRC1.1 is required for menin-inhibitor-induced antileukemia effects. (A) Scatter plots showing gene log2 FC for each gene in 2 biological replicates of CRISPR screen in MOLM-13 cells. (B) MAGeCK results showing ranked genes based on the RRA score of the day 14 VTP50469-treated group compared with the DMSO-treated group in MOLM-13 cells. (C) Western blot analysis showing the levels of the indicated proteins after individual knockout of PRC1.1 complex components BCOR, RYBP, and PCGF1 in OCI-AML2 cells. Two independent sgRNAs were used for targeting each gene. (D-F) Dose-response curves showing the viability of OCI-AML2 cells expressing the indicated sgRNAs, including PCGF1 (D), BCOR (E), and RYBP (F), after a 10-day treatment with the DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. The calculated IC50 values are shown in boxplots. (G) Flow cytometry analysis of the percentages of CD11b+ cells in OCI-AML2 cells expressing sgLuc and sgPCGF1 after a 9-day treatment with DMSO or 1 μM VTP50469. (H) Bar plot depicting the percentages of CD11b+ cells as shown in panel G. (I). Wright-Giemsa stain of OCI-AML2 cells expressing sgLuc and sgPCGF1, after a 9-day treatment with DMSO or 1 μM VTP50469. Scale bar, 10 μm. (J) Flow cytometry analysis of the percentages of CD11b+ cells in OCI-AML2 cells expressing sgLuc and sgBCOR, after a 9-day treatment with DMSO or 1 μM VTP50469. (K) Bar plot depicting the percentages of CD11b+ cells as shown in panel J. (L) Wright-Giemsa stain of OCI-AML2 cells expressing sgLuc and sgBCOR, after a 9-day treatment with DMSO or 1 μM VTP50469. Scale bar, 10 μm. (M-N) Kaplan-Meier survival curve of mice after transplantation of MV4-11 AML cells expressing the indicated sgRNAs. P values were calculated using the log-rank test. IC50, 50% inhibitory concentration; n.s., not significant; SSC, side scatter; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P <. 0001.
PRC1.1 is required for menin-inhibitor-induced antileukemia effects. (A) Scatter plots showing gene log2 FC for each gene in 2 biological replicates of CRISPR screen in MOLM-13 cells. (B) MAGeCK results showing ranked genes based on the RRA score of the day 14 VTP50469-treated group compared with the DMSO-treated group in MOLM-13 cells. (C) Western blot analysis showing the levels of the indicated proteins after individual knockout of PRC1.1 complex components BCOR, RYBP, and PCGF1 in OCI-AML2 cells. Two independent sgRNAs were used for targeting each gene. (D-F) Dose-response curves showing the viability of OCI-AML2 cells expressing the indicated sgRNAs, including PCGF1 (D), BCOR (E), and RYBP (F), after a 10-day treatment with the DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. The calculated IC50 values are shown in boxplots. (G) Flow cytometry analysis of the percentages of CD11b+ cells in OCI-AML2 cells expressing sgLuc and sgPCGF1 after a 9-day treatment with DMSO or 1 μM VTP50469. (H) Bar plot depicting the percentages of CD11b+ cells as shown in panel G. (I). Wright-Giemsa stain of OCI-AML2 cells expressing sgLuc and sgPCGF1, after a 9-day treatment with DMSO or 1 μM VTP50469. Scale bar, 10 μm. (J) Flow cytometry analysis of the percentages of CD11b+ cells in OCI-AML2 cells expressing sgLuc and sgBCOR, after a 9-day treatment with DMSO or 1 μM VTP50469. (K) Bar plot depicting the percentages of CD11b+ cells as shown in panel J. (L) Wright-Giemsa stain of OCI-AML2 cells expressing sgLuc and sgBCOR, after a 9-day treatment with DMSO or 1 μM VTP50469. Scale bar, 10 μm. (M-N) Kaplan-Meier survival curve of mice after transplantation of MV4-11 AML cells expressing the indicated sgRNAs. P values were calculated using the log-rank test. IC50, 50% inhibitory concentration; n.s., not significant; SSC, side scatter; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P <. 0001.
Loss of PRC1.1 leads to epigenetic reactivation of a unique group of noncanonical menin targets upon menin inhibition
To investigate the resistance mechanism in leukemia cells with PRC1.1 loss, we performed transcriptome analysis in PCGF1 wild-type and knockout OCI-AML2 cells treated with either DMSO or VTP50469. Differential gene expression analysis identified 1117 upregulated and 950 downregulated genes (fold change >1.5; adjusted P value <.01) after menin inhibition in wild-type cells (Figure 4A). K-means analysis identified 4 clusters of distinct gene expression patterns (Figure 4A; supplemental Table 3). Notably, cluster 2 genes, primarily associated with hematopoietic lineage development and myeloid differentiation, showed reduced activation by VTP50469 after PCGF1 knockout. Gene-set enrichment analysis (GSEA) further indicated inhibited monocyte differentiation programs in PCGF1 knockout cells after menin inhibition (supplemental Figure 4A). Clusters 3 and 4 represented distinct gene groups that were downregulated by VTP50469. Cluster 3, enriched with MLL fusion oncoprotein targets such as MEIS1, PBX3, and MEF2C, showed consistent repression in both PCGF1 wild-type and knockout cells (Figure 4A-B). In contrast, cluster 4 genes, including MYC, LRP5, and RUNX3, which were linked to the MYC gene signature and ribosome biogenesis, were suppressed in wild-type but not in PCGF1-depleted cells (Figure 4A).
Loss of PRC1.1 leads to epigenetic reactivation of a unique group of noncanonical menin targets upon menin inhibition. (A) K-means clustering of differentially expressed genes in OCI-AML2 cells treated with DMSO or 1 μM VTP50469 for 6 days. Two replicates were used for each group. The list of representative genes and enrichment annotations for each cluster are shown on the right. (B) Normalized read counts for menin-MLL canonical target genes PBX3, MEIS1, and MEF2C in OCI-AML2 cells expressing sgLuc, sgPCGF1, and sgBCOR, followed by a 6-day treatment with DMSO or 1 μM VTP50469. Data were obtained from 2 biological replicates for each group. (C) Chromatin immunoprecipitation (ChIP) enrichment analysis of transcription factor target gene sets in cluster 4 genes as shown in panel A. (D) Heat map showing menin and H2AK119ub ChIP sequencing (ChIP-seq) peaks and their overlaps at transcription start sites of genes in indicated OCI-AML2 cells subjected to a 6-day treatment with DMSO or 1 μM VTP50469. (E) Averaged menin and H2AK119ub ChIP-seq signals over ±3 kb from transcription start sites of menin-specific target genes (left) and menin/H2AK119ub–shared target genes (right) in indicated OCI-AML2 cells subjected to a 6-day treatment with DMSO or 1 μM VTP50469. (F) Schematic illustrating the definition of type I and type II menin target genes. (G) Boxplots showing log2 ratio of menin signal to H2AK119ub signal within ±3 kb of the transcription start sites of type I and II menin target genes. (H) Boxplots illustrating the rescue indices of type I and II menin target genes. A rescue index value of 0 indicates no rescue effect, whereas a value of 1 denotes complete rescue. (I) Scatterplot showing a negative correlation between the rescue index and the signal balance of menin and H2AK119ub, represented by the log2-ratio of menin signal to H2AK119ub signal (top). Boxplots showing the log2FC in gene expression in the indicated OCI-AML2 cells compared with DMSO-treated sgLuc-expressing cells (bottom). Displayed are the top 10% of genes with the most balanced signals (left) and the top 10% of menin-dominant genes. (J) Menin and H2AK119ub ChIP-seq and RNA-seq profiles of representative type I and type II menin targets in indicated OCI-AML2 cells. (K) Schematic models depicting the mechanisms of transcriptional regulation by menin and PRC1.1-mediated H2AK119ub at type I and type II menin target genes. D, DMSO; RNA-seq, RNA sequencing; TSS, transcription start site; V, VTP50469.
Loss of PRC1.1 leads to epigenetic reactivation of a unique group of noncanonical menin targets upon menin inhibition. (A) K-means clustering of differentially expressed genes in OCI-AML2 cells treated with DMSO or 1 μM VTP50469 for 6 days. Two replicates were used for each group. The list of representative genes and enrichment annotations for each cluster are shown on the right. (B) Normalized read counts for menin-MLL canonical target genes PBX3, MEIS1, and MEF2C in OCI-AML2 cells expressing sgLuc, sgPCGF1, and sgBCOR, followed by a 6-day treatment with DMSO or 1 μM VTP50469. Data were obtained from 2 biological replicates for each group. (C) Chromatin immunoprecipitation (ChIP) enrichment analysis of transcription factor target gene sets in cluster 4 genes as shown in panel A. (D) Heat map showing menin and H2AK119ub ChIP sequencing (ChIP-seq) peaks and their overlaps at transcription start sites of genes in indicated OCI-AML2 cells subjected to a 6-day treatment with DMSO or 1 μM VTP50469. (E) Averaged menin and H2AK119ub ChIP-seq signals over ±3 kb from transcription start sites of menin-specific target genes (left) and menin/H2AK119ub–shared target genes (right) in indicated OCI-AML2 cells subjected to a 6-day treatment with DMSO or 1 μM VTP50469. (F) Schematic illustrating the definition of type I and type II menin target genes. (G) Boxplots showing log2 ratio of menin signal to H2AK119ub signal within ±3 kb of the transcription start sites of type I and II menin target genes. (H) Boxplots illustrating the rescue indices of type I and II menin target genes. A rescue index value of 0 indicates no rescue effect, whereas a value of 1 denotes complete rescue. (I) Scatterplot showing a negative correlation between the rescue index and the signal balance of menin and H2AK119ub, represented by the log2-ratio of menin signal to H2AK119ub signal (top). Boxplots showing the log2FC in gene expression in the indicated OCI-AML2 cells compared with DMSO-treated sgLuc-expressing cells (bottom). Displayed are the top 10% of genes with the most balanced signals (left) and the top 10% of menin-dominant genes. (J) Menin and H2AK119ub ChIP-seq and RNA-seq profiles of representative type I and type II menin targets in indicated OCI-AML2 cells. (K) Schematic models depicting the mechanisms of transcriptional regulation by menin and PRC1.1-mediated H2AK119ub at type I and type II menin target genes. D, DMSO; RNA-seq, RNA sequencing; TSS, transcription start site; V, VTP50469.
Next, we performed a chromatin immunoprecipitation enrichment analysis (ChEA) for each gene cluster. Consistent with the gene ontology analysis, cluster 2 genes were enriched with myeloid lineage transcription factors such as SPI1 and IRF8, whereas cluster 4 genes were enriched with MYC family transcription factors and PRC1 component proteins such as KDM2B and RING1B (Figure 4C; supplemental Figure 4B). We then carried out chromatin immunoprecipitation followed by sequencing (ChIP-seq) to determine the genome-wide binding of menin and PRC1.1-catalyzed monoubiquitination of histone H2A at lysine 119 (H2AK119ub). Importantly, the loss of PRC1.1 markedly reduced genome-wide H2AK119ub signals but did not affect the global displacement of menin from chromatin caused by menin inhibition (supplemental Figure 4C-D). Although cluster 3 genes, primarily MLL targets, exhibited a more robust menin binding signal (supplemental Figure 4E), cluster 4 genes showed enrichment of both menin and H2AK119ub binding (supplemental Figure 4F). Importantly, the knockout of PCGF1 markedly reduced H2AK119ub binding of these cluster 4 genes (supplemental Figure 4F), indicating that PRC1.1-mediated H2AK119ub may play a significant role in the gene silencing of cluster 4 genes after menin inhibition.
Further analysis of genome-wide menin and H2AK119ub binding identified 3423 genes that were specifically bound by menin and 1733 genes that were bivalently cobound by both menin and H2AK119ub (Figure 4D; supplemental Table 4). The third group included genes specifically bound by H2AK119ub alone, such as fibroblast growth factor receptor 1 (FGFR1), previously reported as a PRC1.1 target in AML.22 Menin inhibitors effectively removed menin binding from both menin-specific and menin/H2AK119ub–shared targets regardless of PCGF1 depletion (Figure 4E, top). However, the absence of PCGF1 resulted in a significant decrease in the repressive H2AK119ub signals at the menin/H2AK119ub–shared gene targets (Figure 4E, bottom). Next, we focused on 329 menin target genes, defined by menin binding and downregulated upon menin inhibition, and categorized them into 2 groups: type I menin targets (n = 193), bound exclusively by menin, and type II menin targets (n = 136), cobound by both menin and H2AK119ub (Figure 4F). We proposed that type II menin target genes, unlike type I targets dependent solely on menin activation, are regulated by a balance between active menin and repressive H2AK119ub signals. PRC1.1 loss might reduce H2AK119ub at type II targets, countering the repression of menin inhibition. To investigate this, we created a “rescue index” to assess whether gene expression repressed by menin inhibition could return to normal levels in resistant leukemia cells (see Methods for details). An index value of 0 indicates no rescue effect, whereas a value of 1 denotes complete rescue. We found that type II menin target genes exhibited a greater rescue effect on gene expression than type I menin targets (Figure 4G-H). Moreover, the relative enrichment of H2AK119ub compared with menin at menin target sites was a reliable predictor of the extent of this rescue effect (Figure 4I). This was exemplified by type I menin targeting MEIS1, PBX3, and MEF2C and type II menin targeting MYC, LRP5, and RUNX3 (Figure 4J; supplemental Figure 4G). PRC1 and H2AK119ub are known to counteract RNA polymerase II (RNA Pol II) activity.23-25 Interestingly, unlike MEIS1, RNA Pol II continued to bind to the MYC promoter after menin inhibition (supplemental Figure 4H), potentially contributing to active gene transcription in the absence of PRC1-mediated repression. Taken together, we uncovered a noncanonical subset of menin targets that are bivalently marked by both the active menin signal and the repressive H2AK119ub mark. In menin-resistant cells deficient in PRC1.1, although the menin inhibitor effectively removed menin from chromatin and downregulated canonical targets, these noncanonical menin targets underwent epigenetic reactivation due to loss of the H2AK119ub-mediated gene repression (Figure 4K).
Menin inhibitor resistance in PRC1.1-deficient AML cells is mediated by aberrant reactivation of type II menin target MYC
To address the role of type II menin targets in drug resistance, we first performed an unbiased GSEA using the Molecular Signatures Database Transcription Factor Targets gene sets,26 with the goal to identify key transcriptional programs in resistant cells. Remarkably, in all menin inhibitor–treated KMT2A-r AML models, MYC and MYC signature genes were consistently upregulated when PRC1.1 was deficient (Figure 5A-D; supplemental Figure 5A). In addition, menin inhibitors consistently repressed MYC and its signature genes in human KMT2A-r AML cell lines, murine MLL-AF9 leukemia cells, and patient-derived xenograft samples (Figure 5D-E; supplemental Figure 5B-C), suggesting that MYC is a common target of menin in KMT2A-r AML. Intriguingly, we observed a strong positive correlation between the expression levels of the menin-encoding MEN1 gene and MYC by analyzing the Beat AML, The Cancer Genome Atlas (TCGA), and DepMap data sets (Figure 5F), implying that menin might activate MYC in a broader range of AML cell types than those with KMT2A-r.
Menin inhibitor resistance in PRC1.1-deficient AML cells is mediated by aberrant reactivation of type II menin target MYC. (A-C) Volcano plots showing GSEA results using the Molecular Signatures Database Transcription Factor Targets gene sets, comparing VTP50469-treated OCI-AML2 cells with sgPCGF1 (A, left) or sgBCOR (A, right) with sgLuc controls, and similarly for MV4-11 (B) and MOLM-13 (C) cells with sgPCGF1, all under VTP50469 treatment. (D) Bar plots showing the RNA-seq normalized counts of the MYC gene in indicated AML cells. Adjusted P values were calculated using DESeq2. (E) GSEA showing depletion of MYC gene signature in OCI-AML2, MV4-11, MOLM-13, and PDX MLL-AF9 cells (DFAM68555 characterized as MLL-AF9 fusion, MLL3 wild-type AML, and further treated with vehicle [DMSO] or a menin inhibitor [VTP50469]) 11 after menin inhibition treatment. (F) Scatterplot representing the positive correlations between MYC and MEN1 expression levels in samples of patient with AML from the Beat AML and The Cancer Genome Atlas (TCGA) studies, along with AML cell lines from the DepMap database. (G) Schematic diagram displaying the competitive proliferation assay for assessing the effect of MYC constitutive expression on AML cell response to menin inhibition. (H-J) Bar plots showing the percentages of MYC-RFP+ cells in OCI-AML2 (H; 100 nM), MV4-11 (I; 30 nM), and MOLM-13 (J; 10 nM) AML cell lines treated with either DMSO or VTP50469 over specified time periods. (K-M) Bar plots showing the percentages of CD11b+ cells in OCI-AML2 (K), MV4-11 (L), and MOLM-13 (M) AML cell lines with or without MYC overexpression, under treatment with either DMSO or VTP50469 for 6 days. (N) Dose-response curves showing the viability of OCI-AML2 cells expressing the indicated sgRNAs after a 6-day treatment with DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. (O) Dose-response curves showing viabilities of OCI-AML2 cells expressing either sgLuc control, sgPCGF1 alone, or sgPCGF1 combined with sgMYC, after a 9-day treatment with DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. (P) Bar plots showing cell viability of OCI-AML2 cells expressing either sgLuc or sgPCGF1 under 9-day treatment with DMSO, 100 nM VTP50469, 1 nM ABBV075, or a combination of VTP50469 and ABBV075 (combo). (Q) Bar plots showing cell viability of the indicated mouse RN2c cells under a 4-day treatment with DMSO, 100 nM VTP50469, 1 nM ABBV075, or a combination of VTP50469 and ABBV075 (combo). FDR, false discovery rate; NES, normalized enrichment score; ∗∗∗∗P < .0001.
Menin inhibitor resistance in PRC1.1-deficient AML cells is mediated by aberrant reactivation of type II menin target MYC. (A-C) Volcano plots showing GSEA results using the Molecular Signatures Database Transcription Factor Targets gene sets, comparing VTP50469-treated OCI-AML2 cells with sgPCGF1 (A, left) or sgBCOR (A, right) with sgLuc controls, and similarly for MV4-11 (B) and MOLM-13 (C) cells with sgPCGF1, all under VTP50469 treatment. (D) Bar plots showing the RNA-seq normalized counts of the MYC gene in indicated AML cells. Adjusted P values were calculated using DESeq2. (E) GSEA showing depletion of MYC gene signature in OCI-AML2, MV4-11, MOLM-13, and PDX MLL-AF9 cells (DFAM68555 characterized as MLL-AF9 fusion, MLL3 wild-type AML, and further treated with vehicle [DMSO] or a menin inhibitor [VTP50469]) 11 after menin inhibition treatment. (F) Scatterplot representing the positive correlations between MYC and MEN1 expression levels in samples of patient with AML from the Beat AML and The Cancer Genome Atlas (TCGA) studies, along with AML cell lines from the DepMap database. (G) Schematic diagram displaying the competitive proliferation assay for assessing the effect of MYC constitutive expression on AML cell response to menin inhibition. (H-J) Bar plots showing the percentages of MYC-RFP+ cells in OCI-AML2 (H; 100 nM), MV4-11 (I; 30 nM), and MOLM-13 (J; 10 nM) AML cell lines treated with either DMSO or VTP50469 over specified time periods. (K-M) Bar plots showing the percentages of CD11b+ cells in OCI-AML2 (K), MV4-11 (L), and MOLM-13 (M) AML cell lines with or without MYC overexpression, under treatment with either DMSO or VTP50469 for 6 days. (N) Dose-response curves showing the viability of OCI-AML2 cells expressing the indicated sgRNAs after a 6-day treatment with DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. (O) Dose-response curves showing viabilities of OCI-AML2 cells expressing either sgLuc control, sgPCGF1 alone, or sgPCGF1 combined with sgMYC, after a 9-day treatment with DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. (P) Bar plots showing cell viability of OCI-AML2 cells expressing either sgLuc or sgPCGF1 under 9-day treatment with DMSO, 100 nM VTP50469, 1 nM ABBV075, or a combination of VTP50469 and ABBV075 (combo). (Q) Bar plots showing cell viability of the indicated mouse RN2c cells under a 4-day treatment with DMSO, 100 nM VTP50469, 1 nM ABBV075, or a combination of VTP50469 and ABBV075 (combo). FDR, false discovery rate; NES, normalized enrichment score; ∗∗∗∗P < .0001.
To further investigate whether MYC plays a role in contributing menin inhibition resistance, we performed a competitive proliferation assay (Figure 5G). Overexpression of MYC conferred a marked growth advantage and reduced myeloid differentiation under menin inhibition (Figure 5H-M). Conversely, MYC depletion increased sensitivity to menin inhibitor treatment, as evidenced by the substantially reduced 50% inhibitory concentration values (Figure 5N; supplemental Figure 5D-F). Moreover, MYC knockout resensitized PCGF1–deficient AML cells to menin inhibition (Figure 5O; supplemental Figure 5G). Interestingly, our CRISPR screen identified BRD4, a known regulator of MYC,27 as one of the top sensitive hits to menin inhibition (Figure 2A). Therefore, we evaluated the effectiveness of the BRD4 inhibitor, ABBV075,28 in overcoming menin inhibitor resistance. Combining VTP50469 with a relatively low dose of ABBV075 significantly reduced leukemia cell growth in PCGF1-deficient AML cells (Figure 5P-Q; supplemental Figure 5H-I). A similar effect was observed with the MYC inhibitor APTO-25329 (supplemental Figure 5J). Together, these results highlight the crucial role of type II menin targets, such as MYC, in promoting leukemic cell resistance to menin inhibition.
Lastly, FGFR1 has been reported as a PRC1.1 target to promote resistance to tyrosine kinase inhibitors, such as imatinib, in AML cells harboring PRC1.1 mutations.22 In line with this, our data also revealed that FGFR1 was a target of H2AK119ub and was consistently upregulated in AML cells after loss of PRC1.1 (Figure 4D; supplemental Figure 6A-C). However, FGFR1 overexpression did not promote resistance to menin inhibitors (supplemental Figure 6D,F), and FGFR1 knockout did not reverse the resistance to menin inhibition in PCGF1-deficient cells (supplemental Figure 6E,G), indicating the existence of context-specific mechanisms, in which the loss of PRC1.1, contributes to drug resistance in AML.
Loss of PRC1.1 sensitizes AML cells to BCL2 blockade
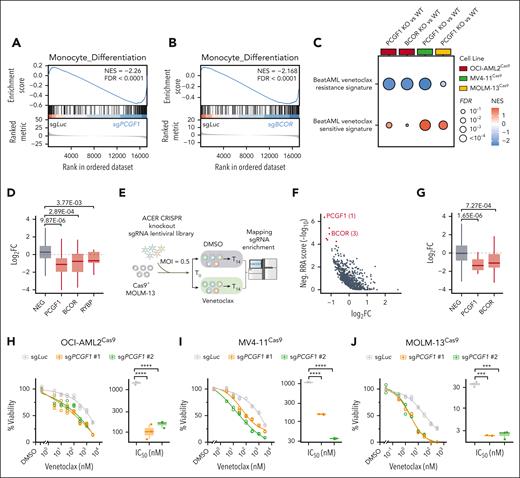
Considering the inherent difficulty in targeting MYC and the clinical complexities associated with bromodomain and extra-terminal domain (BET) inhibitors,30 we next sought to explore novel, actionable strategies for overcoming menin inhibitor resistance. GSEA revealed increased PRC1/2 target gene signatures, reduced myeloid or monocyte differentiation signatures, and elevated primitive lineage gene signatures after PRC1.1 depletion (Figure 6A-B; supplemental Figure 7A-E). Considering that AML cells with reduced monocytic signatures respond better to BCL2 inhibition,31 we hypothesized that PRC1.1-deficient AML cells might be more venetoclax-responsive. By comparing gene signatures associated with venetoclax treatment response, we observed a marked decrease in venetoclax-resistant gene signatures and an increase in venetoclax-sensitive gene signatures in AML cells with the loss of PRC1.1 components (Figure 6C).
Loss of PRC1.1 sensitizes AML cells to BCL2 blockade. (A-B) GSEA showing decreased monocyte differentiation genes in OCI-AML2 cells expressing sgPCGF1 (A) or sgBCOR (B), relative to cells expressing sgLuc. (C) Bubble plot illustrating GSEA results, highlighting the downregulation of Beat AML venetoclax-resistant gene signatures and the upregulation of Beat AML venetoclax-sensitive gene signatures in AML cells with PCGF1 or BCOR knockout, compared with wild-type controls. (D) Boxplots showing the log2 FC in sgRNA counts for specific genes from ACER CRISPR screen in OCI-AML2 cells, comparing venetoclax treatment to DMSO. (E) Schematic overview of the CRISPR knockout screen using the ACER library in MOLM-13 cells treated with either DMSO or venetoclax. (F) Scatterplot showing the log2 FC and negative RRA score from ACER CRISPR screen in MOLM-13 cells, comparing venetoclax treatment to DMSO. (G) Boxplots showing the log2 FC in sgRNA counts for specific genes from ACER CRISPR screen in MOLM-13 cells, comparing venetoclax treatment to DMSO. (H-J) Dose-response curves showing the viability of OCI-AML2 (H), MV4-11 (I), and MOLM-13 (J) cells expressing the indicated sgRNAs, treated for 5, 4, and 3 days, respectively, with either DMSO control or different doses of venetoclax. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. FDR, false discovery rate; NEG, negative control genes; NES, normalized enrichment score; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Loss of PRC1.1 sensitizes AML cells to BCL2 blockade. (A-B) GSEA showing decreased monocyte differentiation genes in OCI-AML2 cells expressing sgPCGF1 (A) or sgBCOR (B), relative to cells expressing sgLuc. (C) Bubble plot illustrating GSEA results, highlighting the downregulation of Beat AML venetoclax-resistant gene signatures and the upregulation of Beat AML venetoclax-sensitive gene signatures in AML cells with PCGF1 or BCOR knockout, compared with wild-type controls. (D) Boxplots showing the log2 FC in sgRNA counts for specific genes from ACER CRISPR screen in OCI-AML2 cells, comparing venetoclax treatment to DMSO. (E) Schematic overview of the CRISPR knockout screen using the ACER library in MOLM-13 cells treated with either DMSO or venetoclax. (F) Scatterplot showing the log2 FC and negative RRA score from ACER CRISPR screen in MOLM-13 cells, comparing venetoclax treatment to DMSO. (G) Boxplots showing the log2 FC in sgRNA counts for specific genes from ACER CRISPR screen in MOLM-13 cells, comparing venetoclax treatment to DMSO. (H-J) Dose-response curves showing the viability of OCI-AML2 (H), MV4-11 (I), and MOLM-13 (J) cells expressing the indicated sgRNAs, treated for 5, 4, and 3 days, respectively, with either DMSO control or different doses of venetoclax. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. FDR, false discovery rate; NEG, negative control genes; NES, normalized enrichment score; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Our ACER CRISPR screen provides an opportunity to assess the impact of epigenetic gene perturbations and their influence on AML cell responses to BCL2 inhibition (Figure 1B). Notably, sgRNAs targeting PRC1.1 genes showed a significant decrease after venetoclax treatment (Figure 6D; supplemental Figure 7F), suggesting that depletion of these genes confers sensitivity to BCL2 inhibition. Moreover, a similar ACER library screen with venetoclax treatment in MOLM-13 cells also revealed the increased sensitivity of AML cells to PRC1.1 subunit depletion, including PCGF1 and BCOR as the top sensitive hits (Figure 6E-G; supplemental Figure 7G). Indeed, PCGF1 loss led to markedly increased venetoclax sensitivity, with 50% inhibitory concentration values showing a significant 10- to 30-fold decrease in 3 KMT2A-r cell lines (Figure 6H-J). To investigate the underlying mechanism, we evaluated BCL2 family genes and additional key regulators of the apoptosis pathway. Although our RNA-sequencing data from 3 KMT2A-r AML cell models did not show a consistent pattern in gene expression, we did observe a trend in which the loss of PRC1.1 resulted in decreased expression of antiapoptotic genes and increased expression of proapoptotic genes (supplemental Figure 7H). Additionally, our analysis of genes differentially regulated by PCGF1 knockout showed that a substantial portion of the upregulated genes (547/744 [73.5%]) were significantly enriched in PRC1.1-catalyzed H2AK119ub binding (the Fisher exact test, P < 2.2e-22; supplemental Figure 7I-J). Analysis of these putative PCGF1 target genes revealed a set of primitive lineage-related genes (supplemental Figure 7K), implying a potential mechanism for the reduced monocytic differentiation program in AML cells with PRC1.1 loss.
Venetoclax effectively suppresses in vivo tumor progression and overcomes menin inhibitor resistance in KMT2A-r leukemia with PCGF1 deficiency
To validate our findings beyond human cell lines, which often have additional genetic changes, we used genetically defined murine AML models. In the RN2 MLL-AF9;NRASG12D model,32Pcgf1 loss resulted in significant resistance to menin inhibition and increased sensitivity to BCL2 blockade (supplemental Figure 8A-C). Similarly, in independently established MLL-AF6 and MLL-AF9 AML cell models from murine hematopoietic stem and progenitor cells, Pcgf1 knockout led to resistance to menin inhibition and enhanced sensitivity to venetoclax (supplemental Figure 8D-I). Consistent with the human model results, menin inhibition repressed Myc expression only in Pcgf1-wild-type, but not in Pcgf1-knockout cells (supplemental Figure 5C). To assess the efficacy of venetoclax in vivo, we transplanted wild-type and Pcgf1 knockout murine MLL-AF6 leukemia cells into immunocompetent mice, followed by treatment with either the vehicle control or a minimal dose of venetoclax (Figure 7A). Venetoclax significantly reduced white blood cell counts and prolonged survival in mice with Pcgf1 knockout leukemia cells (Figure 7B-C). Similarly, xenografts of PCGF1-knockout MV4-11 leukemia cells showed improved responses to venetoclax treatment (Figure 7D-F). Lastly, we demonstrated that venetoclax effectively overcame menin inhibitor resistance in PCGF1-knockout OCI-AML2 cells in vitro (supplemental Figure 8J-K), in the murine MLL-AF6 leukemia model in vivo (Figure 7G-I; supplemental Figure 8L), and in 2 independent KMT2A-r primary AML cell models ex vivo (Figure 7J-L). These results collectively demonstrate that venetoclax effectively inhibits the in vivo progression of KMT2A-r leukemia with PRC1.1 loss and can be used to counteract menin inhibitor resistance caused by PRC1.1 deficiency.
Venetoclax effectively suppresses in vivo tumor progression and overcomes menin inhibitor resistance in KMT2A-r leukemia with PCGF1 deficiency. (A) Schematic showing the transplantation of MLL-AF6 murine AML cells into recipient mice, followed by a 4-week continuous oral treatment starting 5 days after transplantation, using either vehicle control or venetoclax at 20 mg/kg per day. (B) WBC counts in peripheral blood of indicated cohorts, measured 21 days after transplantation. (C) Kaplan-Meier survival curve of mice after transplantation of MLL-AF6 murine AML cells expressing the indicated sgRNAs. The P values were calculated by log-rank test. (D) Schematic showing the transplantation of human MV4-11 MLL-AF4 AML cells into recipient mice, followed by a 7-day continuous oral treatment starting 5 days posttransplantation, using either vehicle control or venetoclax at 75 mg/kg per day. (E) Bar plot showing the percentages of human CD45+ cells in the peripheral blood of indicated cohorts measured 12 days after transplantation. (F) Kaplan-Meier survival curve of mice after transplantation of human MV4-11 AML cells expressing indicated sgRNAs. The P values were calculated by the log-rank test. (G) Schematic showing the transplantation of MLL-AF6 murine AML cells into recipient mice, followed by a 4-week continuous oral treatment starting 5 days after transplantation, using a vehicle control, VTP50469 at 60 mg/kg per day (twice daily ), venetoclax at 20 mg/kg per day, or a combination of VTP50469 and venetoclax. (H-I) The Kaplan-Meier survival curve of mice after transplantation of MLL-AF6 murine AML cells expressing sgRNAs targeting Luc (H) and Pcgf1 (I). The P values were calculated by the log-rank test. (J) Schematic of the competitive growth assay. Primary AML cells were infected with Cas9-EGFP–linked sgRNAs and treated with DMSO, VTP50469, venetoclax, or a combination of both compounds for 10 days, followed by flow cytometry measurement of EGFP+ cells. (K-L) Bar plot showing the relative enrichment of sgRNA+ cells in 2 independent AML primary samples. The percentages of sgRNA+ cells in each treatment were normalized to the DMSO treatment control. (M) Dose-response curves showing viabilities of parental and 2 independent VTP50469-resistant OCI-AML2 (left) and MOLM-13 (right) cells after 9-day and 6-day treatments of VTP50469, respectively. (N) Western blot showing the protein levels of PRC1.1 members PCGF1, BCOR, and RYBP in parental and VTP50469-resistant OCI-AML2 and MOLM-13 cells. Relative FCs in protein levels are labeled below. (O) Dose-response curves showing viabilities of parental and VTP50469-resistant OCI-AML2 (left) and MOLM-13 (right) cells after 6-day and 3-day treatment of venetoclax, respectively. GFP, green fluorescent protein; n.s., not significant; Par, parental; Re, menin inhibitor resistant line; WBC, white blood cells; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
Venetoclax effectively suppresses in vivo tumor progression and overcomes menin inhibitor resistance in KMT2A-r leukemia with PCGF1 deficiency. (A) Schematic showing the transplantation of MLL-AF6 murine AML cells into recipient mice, followed by a 4-week continuous oral treatment starting 5 days after transplantation, using either vehicle control or venetoclax at 20 mg/kg per day. (B) WBC counts in peripheral blood of indicated cohorts, measured 21 days after transplantation. (C) Kaplan-Meier survival curve of mice after transplantation of MLL-AF6 murine AML cells expressing the indicated sgRNAs. The P values were calculated by log-rank test. (D) Schematic showing the transplantation of human MV4-11 MLL-AF4 AML cells into recipient mice, followed by a 7-day continuous oral treatment starting 5 days posttransplantation, using either vehicle control or venetoclax at 75 mg/kg per day. (E) Bar plot showing the percentages of human CD45+ cells in the peripheral blood of indicated cohorts measured 12 days after transplantation. (F) Kaplan-Meier survival curve of mice after transplantation of human MV4-11 AML cells expressing indicated sgRNAs. The P values were calculated by the log-rank test. (G) Schematic showing the transplantation of MLL-AF6 murine AML cells into recipient mice, followed by a 4-week continuous oral treatment starting 5 days after transplantation, using a vehicle control, VTP50469 at 60 mg/kg per day (twice daily ), venetoclax at 20 mg/kg per day, or a combination of VTP50469 and venetoclax. (H-I) The Kaplan-Meier survival curve of mice after transplantation of MLL-AF6 murine AML cells expressing sgRNAs targeting Luc (H) and Pcgf1 (I). The P values were calculated by the log-rank test. (J) Schematic of the competitive growth assay. Primary AML cells were infected with Cas9-EGFP–linked sgRNAs and treated with DMSO, VTP50469, venetoclax, or a combination of both compounds for 10 days, followed by flow cytometry measurement of EGFP+ cells. (K-L) Bar plot showing the relative enrichment of sgRNA+ cells in 2 independent AML primary samples. The percentages of sgRNA+ cells in each treatment were normalized to the DMSO treatment control. (M) Dose-response curves showing viabilities of parental and 2 independent VTP50469-resistant OCI-AML2 (left) and MOLM-13 (right) cells after 9-day and 6-day treatments of VTP50469, respectively. (N) Western blot showing the protein levels of PRC1.1 members PCGF1, BCOR, and RYBP in parental and VTP50469-resistant OCI-AML2 and MOLM-13 cells. Relative FCs in protein levels are labeled below. (O) Dose-response curves showing viabilities of parental and VTP50469-resistant OCI-AML2 (left) and MOLM-13 (right) cells after 6-day and 3-day treatment of venetoclax, respectively. GFP, green fluorescent protein; n.s., not significant; Par, parental; Re, menin inhibitor resistant line; WBC, white blood cells; ∗P < .05; ∗∗P < .01; ∗∗∗P < .001.
To further investigate the link between PRC1.1 loss and resistance to menin inhibition, we developed menin inhibitor-resistant cell lines by exposing cells to gradually increasing doses of menin inhibitor for >2 months (Figure 7M; supplemental Figure 8M). Notably, we observed reduced messenger RNA and protein levels of the PRC1.1 complex subunit BCOR in resistant cells compared to parent cells (Figure 7N; supplemental Figure 8N), suggesting that downregulation of PRC1.1 expression could be associated with acquired menin inhibitor resistance. Moreover, these resistant cells showed a significantly increased sensitivity to venetoclax (Figure 7O; supplemental Figure 8O), further supporting the potential of venetoclax as a therapeutic strategy to counter menin inhibitor resistance in cells with compromised PRC1.1 function.
Discussion
In this study, we report a mechanism regulating the menin inhibitor response that is independent of canonical menin-MLL targets. We identified a specific set of noncanonical menin targets, notably MYC, which is typically suppressed by menin inhibition but remains active in PRC1.1-deficient resistant cells. Unlike canonical menin targets bound solely by menin, these noncanonical targets are transcriptionally controlled by both active menin and the repressive H2AK119ub signals. Moreover, we show that AML cells lacking PRC1.1 undergo transcriptomic changes linked to increased sensitivity to BCL2 blockade, and inhibitors targeting BCL2 can be used to counteract the menin inhibitor resistance seen in PRC1.1-deficient AML cells.
Menin inhibitors have shown promising results in clinical trials but face challenges due to both innate and acquired resistance.8,9 Although menin gene mutations account for ∼40% of resistance cases, the drivers of nongenetic resistance remain to be identified. Intriguingly, in these nongenetic cases, menin inhibitors often continue to effectively suppress canonical menin-MLL target genes,8,12 indicating the sustained on-target effects of menin inhibitors and the presence of alternative resistance mechanisms. A recent study indicated that the accumulation of genetic mutations could lead to menin inhibitor resistance independently of the transcriptional program induced by KMT2A fusion.12 Our research assessed the impact of common gene mutations on menin inhibition response and identified specific mutations, such as those in PRC1.1 components, that were linked to resistance. This indicates that certain AML mutations can affect menin inhibitor effectiveness, guiding the targeted use of these inhibitors in patients with AML. However, it is important to note that the resistance mechanism described herein has yet to be confirmed in patients treated with menin inhibitors, and further clinical-genomic research is needed to understand the full impact of PRC1.1 and its regulated genes on acquired resistance to menin inhibitors in patients with AML.
Our study revealed that menin and PRC1.1 bivalently control the expression of noncanonical menin target genes, however, the precise mechanism that activates target genes in the absence of menin and PRC1.1 remains to be investigated. PRC1.1 complex is known to repress RNA Pol II activity.23-25 One potential hypothesis is that upon menin inhibition, PRC1.1 predominantly represses Pol II activity at these noncanonical targets, with Pol II activity being restored upon depletion of PRC1.1 and removal of H2AK119ub from the promoters of these targets. Further research is required to understand the detailed mechanisms underlying menin and PRC1.1-mediated gene regulation. Through unbiased transcriptomic and epigenomic analyses, our study highlights MYC as one of the key noncanonical menin targets. We demonstrated that pharmacological inhibition of MYC with bromodomain inhibitors could resensitize these cells to menin inhibition. This finding aligns with recent research showing synergistic effects of bromodomain inhibitors and menin inhibition in suppressing AML.33
Our study shows that the loss of PRC1.1 components leads to gene signatures associated with reduced monocytic differentiation and a more primitive state. This is consistent with the previously reported role of PRC1.1 in blocking myeloid differentiation and promoting the expansion of myeloid progenitors in mouse models.34,35 Indeed, our study identified several genes associated with primitive lineages that were bound by H2AK119ub and upregulated after loss of PRC1.1. Further research is needed to elucidate the roles of these potential PRC1.1 targets in lineage specification and their responses to venetoclax. Considering that PRC1.1 component genes, such as BCOR and BCORL1, are mutated in 5% to 10% of myeloid malignancies and are linked to poorer outcomes,22,36,37 it would be intriguing to explore whether these AML could be effectively treated with BCL2 inhibition.
In summary, our study highlights the crucial role of PRC1.1 and its regulated noncanonical menin targets in modulating menin therapy response and suggests potential strategies to enhance menin inhibitor efficacy in PRC1.1-compromised leukemia cells.
Acknowledgments
The authors thank Christopher Vakoc for providing CRISPR-associated protein 9–expressing acute myeloid leukemia cell lines and Ksenia Matlawska-Wasowska for providing retroviral expression constructs for MLL-AF6 and MLL-AF9. The authors thank Lu laboratory members for helpful discussions and technical support. The authors thank the University of Alabama at Birmingham facilities, including the Imaging Core, Flow Cytometry Core, and Animal Studies Core for their professional assistance in this work.
This work was supported in part by the National Institutes of Health, National Cancer Institute (grant R01CA259480 [R.L.]), the Mark Foundation for Cancer Research (R.L.), Gabrielle’s Angel Foundation (R.L.), National Institutes of Health, National Heart, Lung, and Blood Institute (grant R01HL153220 [Y. Zhou]), and the Ontario Institute of Cancer Research (grant IA-133 [K.H.]). R.L. is an American Society of Hematology Scholar in Basic Science and a research scholar of the American Cancer Society (RSG-22-036-01-DMC).
Authorship
Contribution: X.Z. contributed to conceptualization, investigation, methodology, and writing the original draft; L.Z. contributed to data curation and methodology, and wrote the original draft; S.A. contributed to conceptualization, investigation, and writing the original draft; V.V., A.T., C.R., and P.Z. conducted the investigation and wrote, reviewed, and edited the manuscript; S.M., K.J.H., Y. Zhou, and R.B. wrote, reviewed, and edited the manuscript; Y. Zhang and C.C. conducted data curation and wrote, reviewed, and edited the manuscript; and R.L. contributed to conceptualization, supervision, writing the original draft, project administration, formal analysis, and data curation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rui Lu, Division of Hematology & Oncology, Department of Medicine, The University of Alabama at Birmingham, 1824 6th Ave South, WTI 510G, Birmingham, AL; email: ruilu1@uabmc.edu.
References
Author notes
X.Z. and L.Z. contributed equally to this study.
The RNA sequencing (RNA-seq) and chromatin immunoprecipitation–sequencing (ChIP-seq) data are available in the Gene Expression Omnibus database (accession number GSE249080).
Further information and requests for resources, reagents, and scripts for next-generation sequencing data analyses are available on request from the corresponding author, Rui Lu (ruilu1@uabmc.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Menin inhibitor resistance in PRC1.1-deficient AML cells is mediated by aberrant reactivation of type II menin target MYC. (A-C) Volcano plots showing GSEA results using the Molecular Signatures Database Transcription Factor Targets gene sets, comparing VTP50469-treated OCI-AML2 cells with sgPCGF1 (A, left) or sgBCOR (A, right) with sgLuc controls, and similarly for MV4-11 (B) and MOLM-13 (C) cells with sgPCGF1, all under VTP50469 treatment. (D) Bar plots showing the RNA-seq normalized counts of the MYC gene in indicated AML cells. Adjusted P values were calculated using DESeq2. (E) GSEA showing depletion of MYC gene signature in OCI-AML2, MV4-11, MOLM-13, and PDX MLL-AF9 cells (DFAM68555 characterized as MLL-AF9 fusion, MLL3 wild-type AML, and further treated with vehicle [DMSO] or a menin inhibitor [VTP50469]) 11 after menin inhibition treatment. (F) Scatterplot representing the positive correlations between MYC and MEN1 expression levels in samples of patient with AML from the Beat AML and The Cancer Genome Atlas (TCGA) studies, along with AML cell lines from the DepMap database. (G) Schematic diagram displaying the competitive proliferation assay for assessing the effect of MYC constitutive expression on AML cell response to menin inhibition. (H-J) Bar plots showing the percentages of MYC-RFP+ cells in OCI-AML2 (H; 100 nM), MV4-11 (I; 30 nM), and MOLM-13 (J; 10 nM) AML cell lines treated with either DMSO or VTP50469 over specified time periods. (K-M) Bar plots showing the percentages of CD11b+ cells in OCI-AML2 (K), MV4-11 (L), and MOLM-13 (M) AML cell lines with or without MYC overexpression, under treatment with either DMSO or VTP50469 for 6 days. (N) Dose-response curves showing the viability of OCI-AML2 cells expressing the indicated sgRNAs after a 6-day treatment with DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. (O) Dose-response curves showing viabilities of OCI-AML2 cells expressing either sgLuc control, sgPCGF1 alone, or sgPCGF1 combined with sgMYC, after a 9-day treatment with DMSO control or various doses of VTP50469. All cell viabilities were normalized to DMSO treatment. Calculated IC50 values were shown in boxplots. (P) Bar plots showing cell viability of OCI-AML2 cells expressing either sgLuc or sgPCGF1 under 9-day treatment with DMSO, 100 nM VTP50469, 1 nM ABBV075, or a combination of VTP50469 and ABBV075 (combo). (Q) Bar plots showing cell viability of the indicated mouse RN2c cells under a 4-day treatment with DMSO, 100 nM VTP50469, 1 nM ABBV075, or a combination of VTP50469 and ABBV075 (combo). FDR, false discovery rate; NES, normalized enrichment score; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/19/10.1182_blood.2023023644/2/m_blood_bld-2023-023644-gr5.jpeg?Expires=1765882177&Signature=2ji2IsxWZV8qIP~d7yv1aYT6YbivS1GFFg44icdAV-Dyz6u4sJ3mJ4pmzM4icywWqXANLkevtRWGxptZKiqTtv0Zl008Bu3U1zxg2wclQ1ExNC7bdyqXO48g-C1IALa3drUaj3uZXw2-uN70VJUv3-iNuXjMonpeX47Edx8I6UhTbZut13edy14FznneArkVBFGH9khVGG5NtimIZ9-U02swAe8TT~cjsoGGw2J~hUvBsUsxIsh6~UUMa7cLQmQANT6lGY6HrRh6dktLcUxU6z3Prmq0MypBfyZRnTDOCemgtP-XSo0UvQ6l2FHaxCY6bW-mU8KS3XB9XVOCvBSsXA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal