Key Points
Depletion of CD123-redirected CAR T cells with monoclonal antibodies preserves leukemia remission in human AML xenograft models.
AML CAR T-cell depletion enhances feasibility of subsequent allogeneic stem cell transplantation.
Abstract
We and others previously reported potent antileukemia efficacy of CD123-redirected chimeric antigen receptor (CAR) T cells in preclinical human acute myeloid leukemia (AML) models at the cost of severe hematologic toxicity. This observation raises concern for potential myeloablation in patients with AML treated with CD123-redirected CAR T cells and mandates novel approaches for toxicity mitigation. We hypothesized that CAR T-cell depletion with optimal timing after AML eradication would preserve leukemia remission and allow subsequent hematopoietic stem cell transplantation. To test this hypothesis, we compared 3 CAR T-cell termination strategies: (1) transiently active anti-CD123 messenger RNA–electroporated CART (RNA-CART123); (2) T-cell ablation with alemtuzumab after treatment with lentivirally transduced anti–CD123-4-1BB-CD3ζ T cells (CART123); and (3) T-cell ablation with rituximab after treatment with CD20-coexpressing CART123 (CART123-CD20). All approaches led to rapid leukemia elimination in murine xenograft models of human AML. Subsequent antibody-mediated depletion of CART123 or CART123-CD20 did not impair leukemia remission. Time-course studies demonstrated that durable leukemia remission required CAR T-cell persistence for 4 weeks prior to ablation. Upon CAR T-cell termination, we further demonstrated successful hematopoietic engraftment with a normal human donor to model allogeneic stem cell rescue. Results from these studies will facilitate development of T-cell depletion strategies to augment the feasibility of CAR T-cell therapy for patients with AML.
Introduction
Treatment of patients with acute myeloid leukemia (AML) has changed little in the past 40 years, and outcomes are poor; 5-year event-free survival is 20% to 40% in adults and 60% in children.1-5 Therapy-resistant and relapsed AML remain significant sources of cancer mortality, and further intensification of cytotoxic chemotherapy regimens is often futile or poorly tolerated. Allogeneic hematopoietic stem cell transplantation (HSCT) following induction chemotherapy can consolidate leukemia remission and facilitate long-term survival,6,7 although many patients are deemed transplantation ineligible because of persistent disease and/or medical comorbidities.8 Novel therapeutic strategies that are capable of eradicating chemoresistant AML while permitting subsequent HSCT would therefore provide a major advance in the field.
Remarkable progress has been made with the engineering of human T cells with chimeric antigen receptors (CARs) that are redirected against cell surface tumor antigens, and such therapies may provide new immunotherapeutic alternatives to achieve cancer cure.9-11 Dramatic clinical responses have been observed in patients with relapsed/refractory B-cell malignancies treated with CD19-redirected CAR T cells.12-17 Successful development of similar immunotherapies may be particularly beneficial for patients with chemoresistant AML who otherwise lack curative therapies. Preclinical studies have demonstrated the potent antileukemia activity of CAR T cells targeting AML surface proteins, including Lewis-Y, CD33, CD44v6, and CD123 antigens.18-24 Some of these approaches are under early clinical investigation in patients with relapsed/refractory AML25-27 (www.clinicaltrials.gov NCT01864902, NCT02159495, NCT02623582, and NCT02799680).
However, because most AML antigens that have been targeted to date with monoclonal antibodies or CAR T cells are also expressed on normal hematopoietic progenitor cells, on-target/off-tumor myelotoxicity is an expected sequela of anti-AML immunotherapy. Indeed, significant hematologic toxicity of CD33-redirected and CD123-redirected CAR T cells in human AML models has been observed,20,24 which may limit clinical translation of these therapies without subsequent HSCT. As such, myeloid antigen–directed CAR T-cell therapies may therefore be best deployed as novel conditioning regimens prior to transplantation.
In this context, development of effective CAR T-cell depletion strategies after induction of leukemia remission is essential to halt potentially life-threatening toxicities and to enable subsequent HSCT. Various approaches to terminate permanently modified lentiviral or retroviral CAR T cells via incorporated “suicide switches” or to develop “biodegradable” RNA-transfected CAR T cells are thus currently under evaluation.17,28-31 In this study, we compared the efficacy of 3 discrete approaches for T-cell termination: (1) shorter-persisting messenger RNA–modified CD123-redirected CAR T cells (RNA-CART123); (2) lentivirally transduced CD123-redirected CAR T cells (CART123), subsequently depleted with the anti-CD52 monoclonal antibody alemtuzumab; and (3) CART123 coexpressing surface CD20 protein (CART123-CD20), subsequently depleted with the anti-CD20 monoclonal antibody rituximab. We further provide a systematic investigation of the mechanisms and efficacy of antibody-based CAR T-cell depletion, as well as demonstrate successful postdepletion human-to-human HSCT in a xenograft platform. Such T-cell termination strategies may maximize the therapeutic efficacy and overcome potential toxic sequelae of AML CAR T-cell immunotherapy.
Materials and methods
Cell lines and patient specimens
The human AML cell lines MOLM13, MOLM14, MV4-11, and U937 and the Jurkat T-cell line were purchased from the German Collection of Microorganisms and Cell Cultures (Deutsche Sammlung von Mikroorganismen und Zellkulturen) or American Type Culture Collection repositories. All cell lines were verified by short tandem repeat analysis and tested routinely for Mycoplasma contamination. Bioluminescent AML cell lines were created via transduction of firefly luciferase constructs as described.24
Viably cryopreserved primary AML specimens, normal human bone marrow specimens, and normal human T cells were obtained via Institutional Review Board–approved research protocols of the Children’s Hospital of Philadelphia and the University of Pennsylvania, in accordance with the Declaration of Helsinki.
Human AML and normal hematopoiesis xenograft models
Murine xenograft models of human AML were established as described using luciferase-transduced AML cell lines and primary patient specimens.20,24 For cell-line models, 0.5 × 106 to 1 × 106 AML cells per mouse were injected intravenously (IV) into 6- to 8-week-old male or female nonobese diabetic scid γ (NSG) mice (NOD-scid IL2Rγnull; Jackson Laboratories) and monitored for engraftment and therapeutic response by bioluminescent imaging (BLI) as described.20,24 Patient-derived xenograft (PDX) models were established as described,24 via injection of 5 × 106 to 10 × 106 AML cells/mouse into 6- to 8-week-old male or female NOD-scid IL2Rgnull-3/GM/SF (NSGS) mice (Jackson Laboratories) at 24 hours after conditioning with busulfan 30 mg/kg intraperitoneally (IP) (juvenile myelomonocytic leukemia [JMML] 117 and AML290 models; Table 1). Mice were monitored by daily physical examination and weekly retro-orbital venous blood sampling for flow cytometry (FC) analysis of human CD33-phycoerythrin (PE)+ CD45-allophycocyanin (APC)+ CD123-PE-cyanine-7 (Cy7)+ AML and CD3-PacificBlue+ T cells in peripheral blood with CountBright bead quantification (Invitrogen) as described, using Accuri C6, FACSVerse, or LSRII flow cytometers (BD Biosciences).24 FC antibodies were from eBioscience.
Genomic characteristics of primary AML specimens used for PDX model studies
| PDX USI . | Disease status . | Leukemia cytogenetics and molecular lesions . |
|---|---|---|
| AML290 | De novo | 46,XX,der(10)t(8;10)(q11.1;p15);13q LOH KMT2A partial tandem duplication |
| JMML117 | De novo | 45,XY,-7; PTPN11 G503V |
| PDX USI . | Disease status . | Leukemia cytogenetics and molecular lesions . |
|---|---|---|
| AML290 | De novo | 46,XX,der(10)t(8;10)(q11.1;p15);13q LOH KMT2A partial tandem duplication |
| JMML117 | De novo | 45,XY,-7; PTPN11 G503V |
Both AML specimens were CD52 negative by flow cytometry.
LOH, loss of heterozygosity; USI, unique specimen identifier.
Secondary and tertiary PDX models in NSGS mice were created for experimental studies via injection of 2.5 × 106 to 5 × 106 splenocytes from well-engrafted primary xenograft mice (>10% human AML involvement in murine spleens). Once all mice demonstrated >1% human AML in peripheral blood, animals were randomly assigned to experimental treatment (n = 5 to 8 mice per cohort), and assessed by retro-orbital venous bleeding and FC quantification of human AML and T cells. Histopathologic and immunohistochemical analyses of murine tissues for relevant experiments were performed as described.20,24 Normal human hematopoiesis xenograft models were created via injection of 2.5 × 106 normal human bone marrow cells into busulfan-conditioned NSGS mice. Some specimens were T-cell depleted in vitro using CD3 MicroBeads (Miltenyi) prior to injection. All murine studies were conducted according to approved Institutional Animal Care and Use Committee protocols, with adherence to recommended guidelines.32,33
Generation of CD123-redirected CAR T cells
Second-generation CART123 (CD8 hinge and 4-1BB and CD3ζ endodomains) were generated as described.24 The CAR123-CD20 plasmid was created by cloning CD20 into the CAR123 pTRPE plasmid using a P2A system (Figure 1A), with subsequent lentiviral transduction of normal donor T cells as described above (supplemental Data; supplemental Figure 1, available on the Blood Web site). RNA-modified CART123 cells (RNA-CART123) were generated by subcloning of the CAR construct from the pTRPE CAR123 plasmid into a pDA vector,30 followed by in vitro transcription (mMESSAGE mMachineT7 ULTRA; Life Technologies) and RNA purification (RNeasy Mini Kit; Qiagen). T cells were electroporated with the CD123–messenger RNA construct using an ECM830 Square Wave electroporator (Harvard Apparatus BTX) (supplemental Data).30 Surface expression of CAR123 was detected by flow cytometry using a PE-conjugated anti-CD123 peptide antibody.24
Mechanism of rituximab-mediated depletion of CART123-CD20 and alemtuzumab depletion of CART123. (A) Schema of CART123-CD20 construct. (B) Surface expression of CD123-CAR-PE and CD20-PE-Cy7 on CART123-CD20 T cells by FC. (C) In vitro incubation of CD123+ MOLM14 cells with CART123 or CART123-CD20 have similar elevated inflammatory cytokine production as measured by FC, suggesting similar potency of both CD123-redirected CAR T-cell products. Minimal cytokine production occurs with coincubation of CAR T cells and CD123-Jurkat T cells. (D) Similar cytotoxicity is observed with in vitro coculture of MOLM14 cells with CART123 or CART123-CD20 at all effector:target (E:T) ratios, as measured by BLI. (E) Similar cytotoxicity is observed with coculture of MOLM14 cells with RNA-CART123 or CART123-CD20, as measured by BLI. (F) Rituximab depletes CART123-CD20 cells by CDC and by direct cellular cytotoxicity. Coculture of CART123-CD20 with increasing concentrations of rituximab in the absence or presence of 15% complement resulted in CART123-CD20 depletion only in the presence of complement and had direct cytotoxicity at highest concentration. Residual live CAR T cells were quantified by FC after 12 hours of coculture, and percent T-cell depletion was calculated. (G) Rituximab depletes CART123-CD20 by CDC within 4 to 12 hours of coculture. CART123-CD20 cells were incubated with rituximab at indicated concentrations in the presence of complement. Residual live CAR T cells were measured by FC at indicated time points for calculation of T-cell killing. (H) No evidence for ADCC was observed. CART123-CD20 cells (labeled with carboxyfluorescein diacetate succinimidyl ester [CFSE]) were cocultured with Dil-labeled macrophages and rituximab at the indicated concentrations. Phagocytosis after 2 hours (defined as CFSE+Dil+ singlet cells) was quantified by FC. Percent phagocytosis did not differ in the absence or presence of rituximab. (I) Similar to data in panel E, coculture of CART123 cells with alemtuzumab in the absence or presence of complement demonstrates CDC-mediated T-cell killing.
Mechanism of rituximab-mediated depletion of CART123-CD20 and alemtuzumab depletion of CART123. (A) Schema of CART123-CD20 construct. (B) Surface expression of CD123-CAR-PE and CD20-PE-Cy7 on CART123-CD20 T cells by FC. (C) In vitro incubation of CD123+ MOLM14 cells with CART123 or CART123-CD20 have similar elevated inflammatory cytokine production as measured by FC, suggesting similar potency of both CD123-redirected CAR T-cell products. Minimal cytokine production occurs with coincubation of CAR T cells and CD123-Jurkat T cells. (D) Similar cytotoxicity is observed with in vitro coculture of MOLM14 cells with CART123 or CART123-CD20 at all effector:target (E:T) ratios, as measured by BLI. (E) Similar cytotoxicity is observed with coculture of MOLM14 cells with RNA-CART123 or CART123-CD20, as measured by BLI. (F) Rituximab depletes CART123-CD20 cells by CDC and by direct cellular cytotoxicity. Coculture of CART123-CD20 with increasing concentrations of rituximab in the absence or presence of 15% complement resulted in CART123-CD20 depletion only in the presence of complement and had direct cytotoxicity at highest concentration. Residual live CAR T cells were quantified by FC after 12 hours of coculture, and percent T-cell depletion was calculated. (G) Rituximab depletes CART123-CD20 by CDC within 4 to 12 hours of coculture. CART123-CD20 cells were incubated with rituximab at indicated concentrations in the presence of complement. Residual live CAR T cells were measured by FC at indicated time points for calculation of T-cell killing. (H) No evidence for ADCC was observed. CART123-CD20 cells (labeled with carboxyfluorescein diacetate succinimidyl ester [CFSE]) were cocultured with Dil-labeled macrophages and rituximab at the indicated concentrations. Phagocytosis after 2 hours (defined as CFSE+Dil+ singlet cells) was quantified by FC. Percent phagocytosis did not differ in the absence or presence of rituximab. (I) Similar to data in panel E, coculture of CART123 cells with alemtuzumab in the absence or presence of complement demonstrates CDC-mediated T-cell killing.
In vitro T-cell effector function assays
Degranulation and intracellular cytokine production assays
Briefly, CAR T cells were incubated with AML cells at a 1:5 effector:target ratio as described.20 Anti–CD107a-PE-Cy7 (BioLegend), purified anti-CD28 (BD Biosciences), and purified anti-CD49d (BD Biosciences) antibodies and monensin (BD Biosciences) were then added to the cultures. Cells were harvested 4 hours later and stained with fluorophore-conjugated anti–CAR123-Fc, anti-CD3, and anti-CD8 antibodies and Live/Dead Aqua (Invitrogen). Cells were then fixed and permeabilized (Fix/Perm buffers; Invitrogen) and stained with antibodies against interferon γ (eBioscience), tumor necrosis factor α (BioLegend), interleukin 2 (BD Biosciences), granulocyte-macrophage colony-stimulating factor (BD Biosciences), and macrophage inflammatory protein 1-β (BD Biosciences) cytokines for FC analysis.
Cytotoxicity assays
FISH assays
Fluorescence in situ hybridization (FISH) analyses were performed according to standard methodologies at the University of Pennsylvania cytogenetics laboratory. Cells were fixed in acetic acid and methanol, deposited on slides, and stained with fluorescein- or rhodamine-labeled X- and Y-chromosome probes.
In vivo testing of RNA-CART123
NSG mice (n = 10 per treatment) were injected IV with luciferase-expressing MOLM14 (CD123+ AML cell line) cells as described above on day 1, with BLI documentation of engraftment on day 4. Mice were then randomly assigned to treatment with saline, freshly prepared 1 × 107 mock-RNA T cells, or 1 × 107 RNA-CART123 cells IV on days 5, 9, and 16. Cyclophosphamide (Baxter) was administered IP in a single dose of 60 mg/kg for lymphodepletion at 24 hours prior to administration of the second and third RNA-CART123 doses to minimize allogeneic graft-versus-host disease. Some mice were rechallenged with 1 × 106 green fluorescent protein/luciferase–MOLM14 cells after achievement of leukemia remission with RNA-CART123. Animals were followed by BLI and FC for quantification of human AML and CAR T cells.24 Similar studies of RNA-CART123 were performed in engrafted AML PDX models in NSGS mice (n = 8 mice per treatment; 2 PDX models). For PDX studies, human AML cells and T cells were quantified by FC analysis of murine tissues at designated time points.35,36 Animals were humanely euthanized at a preestablished BLI radiance limit (cell-line models) or timing end point (PDX model), or sooner if ill-appearing according to Institutional Animal Care and Use Committee guidelines.
In vivo CAR T-cell elimination with alemtuzumab or rituximab
To assess completeness and to identify optimal timing of antibody-mediated CAR T-cell elimination, mice engrafted with MOLM14 (n = 5 to 8 mice per cohort; week 0 time point) were administered 1 dose of 1 × 106 CART123 IV (week 1 time point). Animals were then treated with 1 dose, 1 or 5 mg/kg IP, of the humanized anti-CD52 antibody alemtuzumab (Genzyme) at 1, 2, or 3 weeks post-CART123 (week 2, 3, or 4 time points). In subsequent experiments, mice engrafted with luciferase-expressing AML cell lines or primary AML cells (Table 1) were treated IV with saline, untransduced T cells (UTD), or CART123 (1 × 106 cells per mouse for cell-line models or 1 × 105 cells per mouse for PDX models). T cells were then depleted with 1 mg/kg alemtuzumab IP at 4 weeks after CART123 (week 5 time point). Animals were assessed weekly by BLI and FC quantification of human AML and CAR T cells, as described above. PDX mice (n = 5 to 8 mice per treatment; n = 2 PDX models) were euthanized at designated time points as depicted in the figures, and murine bone marrow and spleens were harvested for human AML and T-cell quantification by FC. Histopathologic and immunohistochemical analyses of murine tissues were performed in some studies to assess completeness of T-cell ablation with alemtuzumab.
Additional cohorts of MOLM14 (n = 10 per treatment) or PDX mice (n = 5 to 8 per treatment) were treated with saline, UTD, or CART123-CD20 (1 × 106 cells per mouse for cell-line models or 1 × 105 cells per mouse for PDX models) as described above. Animals were subsequently treated with the anti-CD20 antibody rituximab (Roche) at a dose of 1 mg/kg IP at 4 weeks following CART123-CD20 to eliminate T cells. Animals were assessed by BLI and FC to quantify leukemia burden and CAR T cells. PDX mice were sacrificed for end-organ analyses as described above.
For normal hematopoiesis, xenograft experiments (n = 5 mice per cohort), mice engrafted with male-origin healthy donor T-cell–depleted bone marrow mononuclear cells (BMMCs) were treated with saline, autologous UTD, or autologous CART123-CD20, and then treated with 10 μg rituximab IP. Two weeks later, T-cell depletion was confirmed by FC analysis of murine blood. Rituximab-ablated mice were then engrafted with T-cell–depleted female healthy donor BMMCs and assessed by blood FC analysis for 4 weeks prior to bone marrow harvesting for final analysis.
Statistical analyses
Statistical analyses were performed using Prism version 6.0 for Mac or 6.04 for Windows (GraphPad). Comparison of BLI radiance data for in vivo AML cell-line xenograft studies was performed as described using Student t test or 1-way analysis of variance with Holm-Sidak correction for multiple comparisons.20,24 Kaplan-Meier survival curves were compared using the log-rank test. In the figures, asterisks denote statistically significant P values (*≤0.05, **≤0.01, ***≤0.001, and ****≤0.0001), and “ns” indicates lack of statistical significance (P > .05).
Results
Potent in vitro AML cytotoxicity and effective antibody-mediated depletion of CD123-redirected CAR T cells
Lentiviral transduction of human T cells with a CAR123-P2A-CD20 plasmid (Figure 1A) resulted in FC-detectable surface expression of CD123 and CD20 (Figure 1B). CART123-CD20 had in vitro effector function against MOLM14 that was comparable to that of CART123,24 as measured by CD107a degranulation, killing, and cytokine production (Figure 1C-D). These data show that incorporation of surface CD20 into CART123 does not impair anti-AML activity. RNA-CART123 also exhibited comparable in vitro activity to that of CART123-CD20 (Figure 1E).
We then assessed in vitro efficacy of antibody-mediated depletion of CD123-redirected CAR T cells. CART123-CD20 and CART123 were incubated with rituximab and alemtuzumab, respectively, in the absence or presence of rabbit complement, human macrophages, or human peripheral blood mononuclear cells. Rituximab depleted CART123-CD20 cells within 12 hours via complement-mediated cytotoxicity (CDC), although direct cellular cytotoxicity was also observed at highest concentrations (Figure 1F-G). No evidence of antibody-dependent cell-mediated cytotoxicity (ADCC) or phagocytosis was observed with CART123-CD20 coculture with rituximab in the presence of macrophages (Figure 1H) or peripheral blood mononuclear cells (not shown). Similarly, incubation of CART123 with alemtuzumab demonstrated CDC-mediated killing (Figure 1I) and minimal ADCC (not shown).
Alemtuzumab administration after leukemia remission rapidly depletes CART123 cells in vivo
To evaluate the feasibility of alemtuzumab-mediated depletion of CART123, we first sought to exclude direct effects of alemtuzumab upon AML. We detected bright CD52 expression on normal human T lymphocytes and CART123, but dim or negative expression on several human AML cell lines (Figure 2A). These observations suggested that alemtuzumab could kill T cells, but would have minimal AML cytotoxicity. As predicted, in vitro incubation of MOLM14 with various concentrations of alemtuzumab (without or with complement, as shown in Figure 1) did not impair leukemia proliferation versus culture medium–only controls (Figure 2B). Furthermore, treatment of MOLM14 xenograft mice with alemtuzumab 1 mg/kg or 5 mg/kg37,38 had no effect upon in vivo leukemia proliferation, as evidenced by histopathologic analysis of posttreatment murine tissues (Figure 2C).
Effects of alemtuzumab upon human AML. (A) Surface CD52 is minimally expressed on human AML cell lines MOLM13, MOLM14, MV4-11, THP-1, and U937 by FC analysis. Comparably bright CD52 expression is observed on normal human T cells (untransduced) and transduced CART123 cells used in subsequent in vivo experiments. (B) Coculture of MOLM14 cells with alemtuzumab and 15% complement (as in Figure 1) for 72 hours does not impair in vitro leukemia proliferation, as measured by viable cell counting with trypan blue exclusion. Each condition was performed in triplicate. Data points demonstrate mean cell count with standard error of the mean. (C) Histopathologic analyses of NSG mice treated with 1 dose of 1 mg/kg or 5 mg/kg alemtuzumab demonstrate no effect of alemtuzumab upon normal murine hematopoietic tissues (left panels) or upon human AML in NSG mice engrafted with MOLM14 (right panels). Images are hematoxylin and eosin–stained tissue sections from embedded paraffin blocks. Slides were scanned at ×20 magnification with an Aperio Scanscope CS-O slide scanner with visualization via the Aperio Image Analysis Toolkit (Leica Biosystems). ns, not significant by analysis of variance. PBS, phosphate-buffered saline.
Effects of alemtuzumab upon human AML. (A) Surface CD52 is minimally expressed on human AML cell lines MOLM13, MOLM14, MV4-11, THP-1, and U937 by FC analysis. Comparably bright CD52 expression is observed on normal human T cells (untransduced) and transduced CART123 cells used in subsequent in vivo experiments. (B) Coculture of MOLM14 cells with alemtuzumab and 15% complement (as in Figure 1) for 72 hours does not impair in vitro leukemia proliferation, as measured by viable cell counting with trypan blue exclusion. Each condition was performed in triplicate. Data points demonstrate mean cell count with standard error of the mean. (C) Histopathologic analyses of NSG mice treated with 1 dose of 1 mg/kg or 5 mg/kg alemtuzumab demonstrate no effect of alemtuzumab upon normal murine hematopoietic tissues (left panels) or upon human AML in NSG mice engrafted with MOLM14 (right panels). Images are hematoxylin and eosin–stained tissue sections from embedded paraffin blocks. Slides were scanned at ×20 magnification with an Aperio Scanscope CS-O slide scanner with visualization via the Aperio Image Analysis Toolkit (Leica Biosystems). ns, not significant by analysis of variance. PBS, phosphate-buffered saline.
To identify optimal dosing and timing of alemtuzumab-mediated T-cell elimination, we then treated MOLM14-engrafted mice with CART123 (week 1 time point), followed by alemtuzumab at a single dose of 1 mg/kg at weeks 2, 3, or 4. As previously observed,24 CART123 reduced AML below detection levels and facilitated long-term animal survival, whereas control animals had progressive leukemia that resulted in animal death (Figure 3A-B; P < .0001). T-cell depletion prior to complete AML eradication, as expected, allowed leukemia progression in most animals (Figure 3C), providing indirect evidence of successful alemtuzumab-mediated depletion of T cells. Similarly, MOLM14 rechallenge of AML-free mice after T-cell ablation resulted in rapid leukemia progression (Figure 3C). Conversely, MOLM14 rechallenge of AML-free mice that did not undergo CAR T-cell depletion resulted in reexpansion of CAR T cells and subsequent leukemia eradication (Figure 3C), consistent with immunological memory. Taken together, these data demonstrated that CART123 persistence for ≥3 weeks prior to T-cell termination was necessary for long-term AML control in this model.
Serial ablation of CART123 with alemtuzumab in AML xenograft model. NSG mice were injected with luciferase-expressing MOLM14 cells at week 0 time point, then treated with saline, UTD, or CART123 (n = 10 mice per cohort) at week 1 time point. Some CART123-treated mice were subsequently treated with 1 dose of 1 mg/kg alemtuzumab (alem) IP at 1, 2, or 3 weeks following CART123 (denoted as colored bars or as “A”) (weeks 2, 3, or 4 time points; n = 5 to 8 mice per cohort). Animals were assessed by (A) BLI with (B) quantification of radiance as a surrogate for AML burden, then euthanized when moribund. Two CART123-treated animals (red line in panel A) were found dead at >3 months post-T cells without evidence of recurrent leukemia. Animals in CART123-induced “remission” without and with alemtuzumab were subsequently rechallenged with MOLM14. (C) AML burden by BLI and CD3-PacificBlue+ CART123 cells in peripheral blood were quantified during treatment. MOLM14 rechallenge of T-cell–ablated mice at the week 12 time point resulted in rapid AML progression and animal death versus sustained remission of rechallenged non–T-cell–ablated mice with CART123-induced AML remission (****P < .0001 by analysis of variance with log-rank test; denoted for alemtuzumab at week 3 [light green] or week 4 [blue] versus saline control [light blue], and for CART123 [red] versus saline at week 2 or week 3).
Serial ablation of CART123 with alemtuzumab in AML xenograft model. NSG mice were injected with luciferase-expressing MOLM14 cells at week 0 time point, then treated with saline, UTD, or CART123 (n = 10 mice per cohort) at week 1 time point. Some CART123-treated mice were subsequently treated with 1 dose of 1 mg/kg alemtuzumab (alem) IP at 1, 2, or 3 weeks following CART123 (denoted as colored bars or as “A”) (weeks 2, 3, or 4 time points; n = 5 to 8 mice per cohort). Animals were assessed by (A) BLI with (B) quantification of radiance as a surrogate for AML burden, then euthanized when moribund. Two CART123-treated animals (red line in panel A) were found dead at >3 months post-T cells without evidence of recurrent leukemia. Animals in CART123-induced “remission” without and with alemtuzumab were subsequently rechallenged with MOLM14. (C) AML burden by BLI and CD3-PacificBlue+ CART123 cells in peripheral blood were quantified during treatment. MOLM14 rechallenge of T-cell–ablated mice at the week 12 time point resulted in rapid AML progression and animal death versus sustained remission of rechallenged non–T-cell–ablated mice with CART123-induced AML remission (****P < .0001 by analysis of variance with log-rank test; denoted for alemtuzumab at week 3 [light green] or week 4 [blue] versus saline control [light blue], and for CART123 [red] versus saline at week 2 or week 3).
To assess completeness of T-cell eradication with an optimized alemtuzumab schedule, we performed larger studies in CART123-treated AML xenograft mice. Sustained leukemia remission was observed in MOLM14-engrafted animals treated with CART123 at week 1 and with alemtuzumab 4 weeks later (at week 5; representative data in Figure 4A). AML rechallenge of T-cell–ablated mice again resulted in rapid disease progression, indicating successful T-cell elimination by alemtuzumab, whereas mice treated only with CART123 cleared MOLM14 rechallenge (Figure 4A). CART123-treated animals that had achieved leukemia remission prior to T-cell depletion with alemtuzumab remained leukemia free at ≥6 months. Analysis of CART123- and alemtuzumab-treated MOLM14 animals demonstrated complete depletion of CAR T cells in peripheral blood at 24 hours after alemtuzumab administration (Figure 4B) and in bone marrow and spleens at 1 week after alemtuzumab (week 5 time point) (Figure 4C). Alemtuzumab efficacy was further validated in 2 primary (CD52 negative) AML PDX models (representative data in Figure 4D), with sustained leukemia remission at 4 weeks after depletion (Figure 4E). Taken together, these data demonstrate that alemtuzumab-mediated depletion of CAR T cells after leukemia clearance does not impair long-term CART123-induced AML remission and suggest that reengraftment with CD123+ cells (as in HSCT) should be feasible after T-cell depletion.
In vivo termination of CAR T cells with alemtuzumab in human AML xenograft models. (A) MOLM14-bearing NSG mice (n = 10 mice per cohort) were treated IV with saline (gray bar), 1 × 106 UTD (striped bar), or 1 × 106 CART123 (red bars) at week 1 time point and assessed by weekly BLI. CART123 induced AML remission by week 4. One dose of 1 mg/kg alemtuzumab was administered IP at 4 weeks following CART123 (week 5 time point; blue bar) in designated animals for T-cell depletion. MOLM14 rechallenge at week 9 resulted in rapid leukemia progression and animal death only in mice in which CART123 had been previously ablated with alemtuzumab. (B) Representative peripheral blood FC analysis of MOLM14-bearing, CART123-treated mouse before and after alemtuzumab (alem) administration (week 5 time point). No CD3-PacificBlue+ human T cells are detected at 24 hours (h) following a single dose of alemtuzumab. Blue gate denotes human T-cell count in murine blood normalized to quantitative counting beads. Note that fewer T cells remain in peripheral blood at this time point due to prior CART123-mediated AML clearance. (C) Immunohistochemical analysis of CD8+ T cells and CD33+ AML cells in harvested tissues of MOLM14-bearing mice treated with CART123 (week 2, 1 week after CART123) or CART123 with 1 mg/kg alemtuzumab (week 5, 24 hours after alemtuzumab/4 weeks after CART123). Note that animals euthanized at earlier time points in this subexperiment were not included in the main data analysis shown in panel A. (D) FC analyses of human CD45-APC+ CD33-PE+ CD123-PE-Cy7+ AML and CD3-PacificBlue+ CAR T cells in an AML PDX model (juvenile myelomonocytic leukemia [JMML] 117). Rapid T-cell depletion and sustained AML remission were observed in CART123-treated animals following a single dose of alemtuzumab (administered 24 hours prior to week 5 blood sampling). (E) End-study IHC analysis of murine bone marrow from saline and UTD control mice is hypocellular and fibrotic without evidence of residual AML, suggestive of advanced leukemia in these models. Marrow tissues from CART123-treated mice are more cellular and demonstrate AML clearance even after alemtuzumab administration.
In vivo termination of CAR T cells with alemtuzumab in human AML xenograft models. (A) MOLM14-bearing NSG mice (n = 10 mice per cohort) were treated IV with saline (gray bar), 1 × 106 UTD (striped bar), or 1 × 106 CART123 (red bars) at week 1 time point and assessed by weekly BLI. CART123 induced AML remission by week 4. One dose of 1 mg/kg alemtuzumab was administered IP at 4 weeks following CART123 (week 5 time point; blue bar) in designated animals for T-cell depletion. MOLM14 rechallenge at week 9 resulted in rapid leukemia progression and animal death only in mice in which CART123 had been previously ablated with alemtuzumab. (B) Representative peripheral blood FC analysis of MOLM14-bearing, CART123-treated mouse before and after alemtuzumab (alem) administration (week 5 time point). No CD3-PacificBlue+ human T cells are detected at 24 hours (h) following a single dose of alemtuzumab. Blue gate denotes human T-cell count in murine blood normalized to quantitative counting beads. Note that fewer T cells remain in peripheral blood at this time point due to prior CART123-mediated AML clearance. (C) Immunohistochemical analysis of CD8+ T cells and CD33+ AML cells in harvested tissues of MOLM14-bearing mice treated with CART123 (week 2, 1 week after CART123) or CART123 with 1 mg/kg alemtuzumab (week 5, 24 hours after alemtuzumab/4 weeks after CART123). Note that animals euthanized at earlier time points in this subexperiment were not included in the main data analysis shown in panel A. (D) FC analyses of human CD45-APC+ CD33-PE+ CD123-PE-Cy7+ AML and CD3-PacificBlue+ CAR T cells in an AML PDX model (juvenile myelomonocytic leukemia [JMML] 117). Rapid T-cell depletion and sustained AML remission were observed in CART123-treated animals following a single dose of alemtuzumab (administered 24 hours prior to week 5 blood sampling). (E) End-study IHC analysis of murine bone marrow from saline and UTD control mice is hypocellular and fibrotic without evidence of residual AML, suggestive of advanced leukemia in these models. Marrow tissues from CART123-treated mice are more cellular and demonstrate AML clearance even after alemtuzumab administration.
Antileukemia efficacy of RNA-CART123
We next assessed in vivo activity of RNA-CART123 in AML models. MOLM14-engrafted mice treated with 3 doses of RNA-CART123 demonstrated rapid AML clearance (Figure 5A) and remained in remission for >6 months. MOLM14 rechallenge at 8 weeks following RNA-CART123 resulted in rapid leukemia progression (Figure 5A), confirming shorter persistence of RNA-modified CAR T cells. RNA-CART123 similarly eradicated leukemia in 2 PDX models, as measured by human AML quantification in murine tissues at 6 weeks post-T cells (Figure 5B), with no detectable RNA-CART123 by FC analysis (not shown). These data demonstrate potent anti-AML efficacy of RNA-CART123, but also its potential to limit hematologic toxicity given its shorter in vivo persistence.
Anti-AML efficacy of RNA-CART123 and of CART123/CD20 with rituximab depletion. (A) MOLM14-bearing NSG mice (n = 10 mice per cohort) were treated IV with saline, mock-RNA T cells × 3 doses, RNA-CART123 × 3 doses, CART123, or CART123-CD20 and assessed by BLI. Some mice were injected IP with 60 mg/kg cyclophosphamide diluted in 200 μL saline at 24 hours prior to second and third doses of mock-RNA T cells or RNA-CART123 to deplete previously administered T cells. RNA-CART123 and CART123-CD20 eradicated leukemia by 4 weeks post-T cells (week 5 time point), with similar kinetics to those of CART123, resulting in long-term animal survival. Ablation of CART123/CD20 with 1 mg/kg rituximab IP administered at week 5 did not alter leukemia remission status. MOLM14 rechallenge at week 9 resulted in rapid disease progression and animal death in mice previously treated with RNA-CART123, confirming lack of longer-term persistence of these T cells in vivo. MOLM14 rechallenge of CART123-CD20–treated animals subsequently treated with rituximab also resulted in rapid AML relapse, confirming successful prior ablation of CART123-CD20 in vivo. (B) Treatment of AML290-PDX mice (established as in Figure 4D; n = 8 mice per cohort) with 3 doses of RNA-CART123, as in panel A, induced human leukemia remission measured in murine tissues harvested at 6 weeks after the third dose of T cells (week 8 time point). (C) Treatment of juvenile myelomonocytic leukemia [JMML] 117 PDX mice with CART123 (123) or CART123-CD20 (123-CD20) at week 1 (n = 5 to 8 mice per cohort) resulted in marked reduction in CD45-APC+ CD33-PE+ CD123-PE-Cy7+ AML burden in murine tissues at 8 weeks post-T cells (week 9 time point). PDX mice treated with 1 dose of 1 mg/kg rituximab at the week 5 time point demonstrated sustained AML remission at the week 9 time point (8 weeks after CART123-CD20, 4 weeks after rituximab). (D) FC analysis of CD45-APC+ CD3-PacificBlue+ CAR T cells in peripheral blood of JMML117 PDX mice treated with CART123-CD20 without and with subsequent rituximab. Total CAR T-cell counts (blue gate) in murine blood normalized by quantitative counting beads are markedly reduced following rituximab treatment, although T-cell elimination is less rapid than with alemtuzumab treatment, as in Figure 3. Note that detectable CART123-CD20 decreased over time due to ongoing AML clearance from peripheral blood. (E) Immunohistochemical (IHC) staining of harvested murine spleen tissues, as in panel C, for CD33+ human AML and CD3+ CAR T cells at week 9 time point (8 weeks post-T cells) in JMML117 PDX model. Slides were scanned at ×20 magnification with an Aperio Scanscope CS-O slide scanner with visualization via the Aperio Image Analysis Toolkit (Leica Biosystems).
Anti-AML efficacy of RNA-CART123 and of CART123/CD20 with rituximab depletion. (A) MOLM14-bearing NSG mice (n = 10 mice per cohort) were treated IV with saline, mock-RNA T cells × 3 doses, RNA-CART123 × 3 doses, CART123, or CART123-CD20 and assessed by BLI. Some mice were injected IP with 60 mg/kg cyclophosphamide diluted in 200 μL saline at 24 hours prior to second and third doses of mock-RNA T cells or RNA-CART123 to deplete previously administered T cells. RNA-CART123 and CART123-CD20 eradicated leukemia by 4 weeks post-T cells (week 5 time point), with similar kinetics to those of CART123, resulting in long-term animal survival. Ablation of CART123/CD20 with 1 mg/kg rituximab IP administered at week 5 did not alter leukemia remission status. MOLM14 rechallenge at week 9 resulted in rapid disease progression and animal death in mice previously treated with RNA-CART123, confirming lack of longer-term persistence of these T cells in vivo. MOLM14 rechallenge of CART123-CD20–treated animals subsequently treated with rituximab also resulted in rapid AML relapse, confirming successful prior ablation of CART123-CD20 in vivo. (B) Treatment of AML290-PDX mice (established as in Figure 4D; n = 8 mice per cohort) with 3 doses of RNA-CART123, as in panel A, induced human leukemia remission measured in murine tissues harvested at 6 weeks after the third dose of T cells (week 8 time point). (C) Treatment of juvenile myelomonocytic leukemia [JMML] 117 PDX mice with CART123 (123) or CART123-CD20 (123-CD20) at week 1 (n = 5 to 8 mice per cohort) resulted in marked reduction in CD45-APC+ CD33-PE+ CD123-PE-Cy7+ AML burden in murine tissues at 8 weeks post-T cells (week 9 time point). PDX mice treated with 1 dose of 1 mg/kg rituximab at the week 5 time point demonstrated sustained AML remission at the week 9 time point (8 weeks after CART123-CD20, 4 weeks after rituximab). (D) FC analysis of CD45-APC+ CD3-PacificBlue+ CAR T cells in peripheral blood of JMML117 PDX mice treated with CART123-CD20 without and with subsequent rituximab. Total CAR T-cell counts (blue gate) in murine blood normalized by quantitative counting beads are markedly reduced following rituximab treatment, although T-cell elimination is less rapid than with alemtuzumab treatment, as in Figure 3. Note that detectable CART123-CD20 decreased over time due to ongoing AML clearance from peripheral blood. (E) Immunohistochemical (IHC) staining of harvested murine spleen tissues, as in panel C, for CD33+ human AML and CD3+ CAR T cells at week 9 time point (8 weeks post-T cells) in JMML117 PDX model. Slides were scanned at ×20 magnification with an Aperio Scanscope CS-O slide scanner with visualization via the Aperio Image Analysis Toolkit (Leica Biosystems).
CART123-CD20 cells eradicate AML in xenograft models and can be depleted by rituximab
Treatment of MOLM14 mice with CART123-CD20 (week 1 time point) induced rapid leukemia clearance (Figure 5A) and near-complete eradication of human AML in PDX models (Figure 5C), demonstrating that incorporation of CD20 into the CAR123 construct did not appreciably impair the antileukemia efficacy of CART123. Subsequent treatment of AML PDX mice with rituximab at a single dose of 1 mg/kg at 4 weeks following CART123-CD20 (week 5 time point) resulted in T-cell depletion in peripheral blood and other tissues (Figure 5D-E), although T-cell clearance appeared less rapid than with alemtuzumab (Figure 4B).
To confirm T-cell eradication, rituximab-ablated mice were rechallenged with AML at 8 weeks post-T cells (week 9 time point). Mice treated with CART123-CD20 alone rejected tumor, whereas animals in which T cells had been ablated with rituximab had progressive leukemia (Figure 5A). Results from these studies demonstrate an additional CD123-targeting approach for AML, with subsequent CAR T-cell depletion that does not compromise leukemia remission.
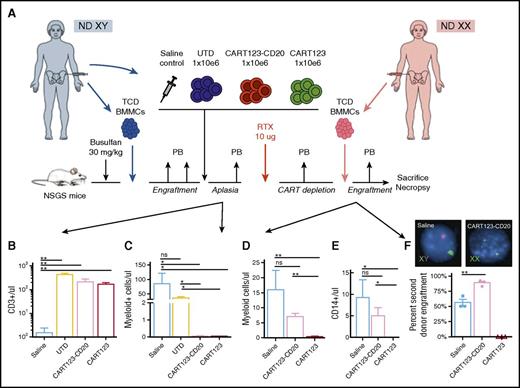
Termination of CD123-redirected CAR T cells allows rescue HSCT
To determine feasibility of HSCT after CAR T-cell depletion, we treated mice engrafted with normal male donor BMMCs with saline or control UTD, CART123-CD20, or CART123 (Figure 6A) and confirmed detection of T cells in peripheral blood of treated animals (Figure 6B). CART123-CD20 administration resulted in expected myelotoxicity, with significant depletion of human CD3−/CD19− cells in murine blood versus control animals (Figure 6C), as reported.24 Mice were then treated with rituximab to deplete CAR T cells, and then injected with normal female donor BMMCs to model HSCT. Three weeks later, FC analysis of murine blood confirmed human myeloid engraftment only in animals initially treated with CART123-CD20 and depleted with rituximab, but not with CART123 (Figure 6D). Female donor BMMCs also differentiated into neutrophils (not shown) and monocytes (Figure 6E) and were detectable in peripheral blood at 3 weeks after injection. FISH analysis of bone marrow at the study end point confirmed engraftment of only female-origin cells in CART123-CD20–treated/rituximab–ablated mice (Figure 6F) versus mixed male- and female-origin engraftment in control animals and rejection of female BMMCs in CART123-treated animals. These data thus demonstrate anticipated on-target/off-tumor hematologic toxicity of CD123-targeted CAR T-cell immunotherapy, as well as the ability to restore normal hematopoiesis after CART123-CD20 depletion with rituximab.
Effective termination of CART123 allows subsequent engraftment of human bone marrow cells. (A) Experimental schema: NSGS mice were conditioned with busulfan 30 mg/kg IP prior to IV injection of 2.5 × 106 T-cell–depleted (TCD) BMMCs from a healthy normal male donor (ND). Human hematopoietic engraftment was confirmed 2 weeks later by FC analysis of murine peripheral blood (PB). CD123-redirected CAR T cells were generated from the same donor. Xenografted mice were treated with 1 dose of saline, UTD, CART123, or CART123-CD20 IV (n = 5 mice per cohort). Two weeks following T cells, mice were bled to confirm reduction/ablation of myeloid cells by CART123-CD20. Mice subsequently received 1 dose of 10 μg rituximab (RTX) IP to deplete CAR T cells and were assessed by serial peripheral blood analysis to confirm eradication of T cells. Mice were then injected with 2.5 × 106 T-cell–depleted BMMCs IV from a healthy female donor. Four weeks after infusion of the female-origin bone marrow, mice were euthanized and bone marrow was harvested for analysis of tissues. (B) Analysis of murine peripheral blood 2 weeks after T-cell treatment demonstrates significant expansion of CD3+ T cells in mice treated with CART123, CART123-CD20, or UTD, but not with saline. (C) Analysis of murine peripheral blood 2 weeks after T-cell treatment demonstrates significant reduction of human CD3-PE-Cy7/CD19-APC double-negative myeloid cells in mice treated with CART123-CD20 or CART123 versus UTD or saline controls. (D) At study end point (3 weeks following injection of female BMMCs), xenograft mice treated with CART123-CD20 and rituximab ablation demonstrate peripheral blood engraftment of myeloid cells and differentiation into monocytes (E), whereas mice treated with CART123 cells rejected engraftment of female donor BMMCs. (F) At study end point, FISH analysis of bone marrow from xenograft mice treated with CART123-CD20 shows engraftment of second donor female-origin (XX) hematopoietic engraftment. Conversely, mixed male-origin (XY) and female-origin hematopoietic engraftment is observed in saline-treated control mice, and previously, CART123-treated animals rejected second transplantation with female BMMCs. Data are representative of 2 independent experiments.
Effective termination of CART123 allows subsequent engraftment of human bone marrow cells. (A) Experimental schema: NSGS mice were conditioned with busulfan 30 mg/kg IP prior to IV injection of 2.5 × 106 T-cell–depleted (TCD) BMMCs from a healthy normal male donor (ND). Human hematopoietic engraftment was confirmed 2 weeks later by FC analysis of murine peripheral blood (PB). CD123-redirected CAR T cells were generated from the same donor. Xenografted mice were treated with 1 dose of saline, UTD, CART123, or CART123-CD20 IV (n = 5 mice per cohort). Two weeks following T cells, mice were bled to confirm reduction/ablation of myeloid cells by CART123-CD20. Mice subsequently received 1 dose of 10 μg rituximab (RTX) IP to deplete CAR T cells and were assessed by serial peripheral blood analysis to confirm eradication of T cells. Mice were then injected with 2.5 × 106 T-cell–depleted BMMCs IV from a healthy female donor. Four weeks after infusion of the female-origin bone marrow, mice were euthanized and bone marrow was harvested for analysis of tissues. (B) Analysis of murine peripheral blood 2 weeks after T-cell treatment demonstrates significant expansion of CD3+ T cells in mice treated with CART123, CART123-CD20, or UTD, but not with saline. (C) Analysis of murine peripheral blood 2 weeks after T-cell treatment demonstrates significant reduction of human CD3-PE-Cy7/CD19-APC double-negative myeloid cells in mice treated with CART123-CD20 or CART123 versus UTD or saline controls. (D) At study end point (3 weeks following injection of female BMMCs), xenograft mice treated with CART123-CD20 and rituximab ablation demonstrate peripheral blood engraftment of myeloid cells and differentiation into monocytes (E), whereas mice treated with CART123 cells rejected engraftment of female donor BMMCs. (F) At study end point, FISH analysis of bone marrow from xenograft mice treated with CART123-CD20 shows engraftment of second donor female-origin (XX) hematopoietic engraftment. Conversely, mixed male-origin (XY) and female-origin hematopoietic engraftment is observed in saline-treated control mice, and previously, CART123-treated animals rejected second transplantation with female BMMCs. Data are representative of 2 independent experiments.
Discussion
Outcomes for patients with relapsed or chemotherapy-refractory AML are dismal, and alternative treatments are necessary to improve cure. Considerable enthusiasm thus exists for new immunotherapeutic approaches, particularly for patients with chemoresistant disease. Progress has been hampered by lack of identified tumor-specific antigens and potential on-target/off-tumor effects in normal tissues. However, some antibody-based immunotherapies have demonstrated clinical activity with acceptable toxicity profiles. For example, the addition of the CD33-targeting antibody-drug conjugate gemtuzumab ozogamicin to chemotherapy improved event-free survival, particularly in patients whose leukemias had highest CD33 expression.6,39-41 Early-phase clinical trials of other CD33–antibody-drug conjugate therapies such as vadastuximab talirine (SGN-33A) are also underway (NCT01902329, NCT02326584) on the basis of favorable anti-AML activity demonstrated in preclinical studies.42 Bispecific T-cell–engaging or dual-affinity–retargeting (DART) antibody therapies that exert cytotoxicity via linkage of patients’ endogenous T cells with tumor cells, such as the CD3 × CD123 DART MGD006, have also demonstrated promising anti-AML activity.43-45 MGD006 and other CD123-targeting antibodies are currently under phase 1 clinical investigation (NCT02152956, NCT01632852, NCT02715011, and NCT02472145).
Given the apparent tolerability and efficacy of antibody-based therapies, it is plausible that similarly-targeted antigen-redirected CAR T-cell immunotherapies will also have robust anti-AML activity. However, CAR T cells are “living drugs” with potential for exponential in vivo expansion and long-term persistence, which may evoke unique antileukemia potency together with significant toxicities that have not been observed in patients treated with antibody therapies.9 Indeed, CD19-redirected CAR T-cell persistence in patients with B-cell lymphoblastic leukemia, with consequent normal B-lymphocyte aplasia for months to years, has been reported.15 Although B-cell aplasia appears clinically tolerable and is treatable with immune globulin replacement,46,47 the anticipated on-target/off-tumor myeloablation induced by AML CAR T-cell immunotherapies may be life threatening. We previously demonstrated significant depletion of normal hematopoietic cells in preclinical AML models treated with permanently modified CD33- or CD123-redirected CAR T cells.20,24 Two recent reports of adults with relapsed AML treated with CD33 or CD123 CAR T cells describe transient antileukemia efficacy and consequent pancytopenia,26 ,27 further suggesting need for CAR T-cell depletion and/or HSCT rescue.
AML CAR T-cell termination may indeed be required for successful HSCT, given that T-cell persistence may mediate rejection of infused hematopoietic stem cells. It is currently unknown whether conditioning regimens for patients with AML are capable of completely depleting CAR T cells prior to HSCT. Depletion via genetic suicide switches or administration of multiple infusions of shorter-lived RNA-modified CAR T cells are thus attractive strategies for AML immunotherapy. Several genetic approaches have been developed, including incorporation of herpes simplex virus thymidine kinase, inducible caspase 9, or truncated epidermal growth factor receptor into the CAR construct.48 Some of these approaches have proven quite immunogenic in patients (eg, herpes simplex virus thymidine kinase) or require administration of T-cell suicide–inducing drugs to the patient to terminate CAR T cells.17,22,28,49-52 Other approaches initially developed to decrease complications of graft-versus-host disease after HSCT include synthetic extracellular CD20 expression on T cells for elimination with anti-CD20 antibodies.31,45,53-55
Here we report several preclinical approaches to increase the therapeutic index of CD123-redirected AML CAR T-cell immunotherapy that are imminently translatable to the clinic. First, we show that long-term AML remission in preclinical models is achievable with shorter-persisting RNA-CART123. Second, we demonstrate that a single low dose of alemtuzumab efficiently and completely eliminates human CAR-modified T cells, and that CART123 depletion after leukemia clearance facilitates sustained remission and survival. Third, we report that modification of CART123 with surface CD20 (CART123-CD20) does not impair anti-AML efficacy and allows successful CAR T-cell depletion with rituximab. Finally, we demonstrate that CAR T-cell ablation in normal human hematopoiesis xenograft models allows subsequent HSCT rescue. We further identify CDC as the primary mechanism of antibody-mediated depletion of CD123-redirected CAR T cells, which is important in the setting of CAR T-cell–induced myelosuppression, whereby decreased numbers of (previously CART123 depleted) CD123+ effector cells may limit ADCC capabilities.37,56 Taken together, these findings establish the robust antileukemia efficacy of 3 CD123 CAR T-cell immunotherapy approaches with potential toxicity mitigation via shorter persistence or antibody-mediated termination of T cells. Although our studies did not aim to establish superiority of 1 termination approach, alemtuzumab-mediated depletion of CART123 appeared more effective than rituximab-mediated depletion of CART123-CD20 in our models. In addition, RNA-CART123 is an attractive therapeutic approach for clinical translation, given its confirmed potent anti-AML activity as well as its apparent shorter persistence.
These studies have several important limitations that require consideration. Although immunocompromised murine xenograft models provide a robust preclinical platform for efficient in vivo evaluation of novel therapeutics, our AML-only or normal hematopoiesis–only models cannot fully predict the potential for simultaneous on-target/on-tumor leukemia cytotoxicity and on-target/off-tumor bystander toxicity.43 Furthermore, whereas the AML rechallenge experiments imply successful antecedent depletion of CD123-redirected CAR T cells, we were unable to prove definitively in our preclinical xenograft models that subsequent allogeneic transplantation would not be compromised by prior CAR T-cell immunotherapy, even with T-cell depletion. Determination of the efficacy of CD123 CAR T cells and the potential success or failure of HSCT rescue thus may only be truly accomplished in the context of early-phase clinical trial evaluation. In addition, although alemtuzumab or rituximab eliminated CAR T cells in our mouse models, the severity and duration of consequent lymphodepletion that may occur in treated patients is unknown and may confer additional infectious risk. Determination of the lowest effective clinical dosing of these antibodies will be necessary to optimize CAR T-cell depletion without causing undue infectious risk in the setting of (1) neutropenia from underlying leukemia and from anti-AML CAR T-cell immunotherapy and (2) B lymphopenia and T lymphopenia from alemtuzumab, or B lymphopenia from rituximab ablation of T cells.
Reliable, complete T-cell termination after induction of leukemia remission may be a critical factor for successful clinical translation of anti-AML CAR T-cell immunotherapies. Indeed, 2 phase 1 trials of CD123-redirected CAR T cells employ suicide strategies: the NCT02623582 trial at the University of Pennsylvania is investigating the safety and preliminary efficacy of an RNA-CART123 approach, whereas the NCT02159495 trial at the City of Hope Medical Center is testing lentiviral CART123–truncated epidermal growth factor receptor with potential subsequent cetuximab administration. Here, we demonstrate robust preclinical activity of RNA-CART123 that informed development of the NCT02623582 trial. We additionally report potent activity of 2 T-cell depletion strategies with alemtuzumab and rituximab, which are commonly and safely used in patients with hematologic malignancies. We predict that CAR T-cell ablation can augment the efficacy of these new immunotherapies and may facilitate subsequent HSCT rescue, but definitive testing of this hypothesis will ultimately require careful and rigorous clinical evaluation in patients with AML.
Presented in part at the 57th annual meeting of the American Society of Hematology, Orlando, FL, 5-8 December, 2015; and the 4th International Conference on Immunotherapy in Pediatric Oncology, Seattle, WA, 25-26 September 2015.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank William Fix and Daniel Martinez for assistance with animal studies and histopathologic analyses.
This work was supported by the National Institutes of Health, National Cancer Institute (K08CA184418 [S.K.T.], K08CA194256 [S.G.], and K12CA090628 [S.S.K.]), Cookies for Kids’ Cancer Foundation (S.K.T.), Press On Foundation (S.K.T.), Andrew McDonough B+ Foundation (S.K.T.), Gabrielle’s Angel Foundation for Cancer Research (S.K.T.), Stand Up to Cancer–St. Baldrick’s Foundation Pediatric Dream Team Translational Research Grant (SU2C-AACR-DT1113) (S.K.T. and S.A.G.), Predolin Foundation (S.S.K.), and a research agreement with Novartis Pharmaceuticals (S.S.K., M.R., C.H.J., S.A.G., and S.G.). Stand Up to Cancer is a program of the Entertainment Industry Foundation administered by the American Association for Cancer Research. S.K.T. was an Alex’s Lemonade Stand Foundation Center of Excellence Scholar in Developmental Therapeutics. S.S.K. is a Mayo Clinic Scholar. S.G. was an American Society of Hematology Clinical Research Fellow Scholar.
Authorship
Contribution: S.K.T. and S.S.K. designed and oversaw studies, conducted experiments, analyzed and interpreted data, and wrote the manuscript; F.S., M.R., O.S., M.K., Y.L., and A.S.-H. performed experiments and analyzed data; J.J.D.M., M.C., S.A.G., and C.H.J. provided key experimental reagents and interpreted data; S.G. oversaw studies, analyzed and interpreted data, and edited the manuscript; all authors critically reviewed the manuscript prior to submission.
Conflict-of-interest disclosure: S.S.K., M.R., O.S., M.K., A.S.-H., S.A.G., C.H.J., and S.G. receive research funding from Novartis Pharmaceuticals (NVP) and/or work under a University of Pennsylvania–NVP research alliance. S.S.K., M.R., C.H.J., S.A.G., and S.G. hold patents related to CAR T-cell immunotherapy technologies, which are managed under the University of Pennsylvania conflict-of-interest policy. S.A.G. consults for NVP. The remaining authors declare no competing financial interests.
Correspondence: Sarah K. Tasian, Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, CTRB 3010, Philadelphia, PA 19104; e-mail: tasians@email.chop.edu.
References
Author notes
S.K.T. and S.S.K. contributed equally to this study.

![Figure 1. Mechanism of rituximab-mediated depletion of CART123-CD20 and alemtuzumab depletion of CART123. (A) Schema of CART123-CD20 construct. (B) Surface expression of CD123-CAR-PE and CD20-PE-Cy7 on CART123-CD20 T cells by FC. (C) In vitro incubation of CD123+ MOLM14 cells with CART123 or CART123-CD20 have similar elevated inflammatory cytokine production as measured by FC, suggesting similar potency of both CD123-redirected CAR T-cell products. Minimal cytokine production occurs with coincubation of CAR T cells and CD123-Jurkat T cells. (D) Similar cytotoxicity is observed with in vitro coculture of MOLM14 cells with CART123 or CART123-CD20 at all effector:target (E:T) ratios, as measured by BLI. (E) Similar cytotoxicity is observed with coculture of MOLM14 cells with RNA-CART123 or CART123-CD20, as measured by BLI. (F) Rituximab depletes CART123-CD20 cells by CDC and by direct cellular cytotoxicity. Coculture of CART123-CD20 with increasing concentrations of rituximab in the absence or presence of 15% complement resulted in CART123-CD20 depletion only in the presence of complement and had direct cytotoxicity at highest concentration. Residual live CAR T cells were quantified by FC after 12 hours of coculture, and percent T-cell depletion was calculated. (G) Rituximab depletes CART123-CD20 by CDC within 4 to 12 hours of coculture. CART123-CD20 cells were incubated with rituximab at indicated concentrations in the presence of complement. Residual live CAR T cells were measured by FC at indicated time points for calculation of T-cell killing. (H) No evidence for ADCC was observed. CART123-CD20 cells (labeled with carboxyfluorescein diacetate succinimidyl ester [CFSE]) were cocultured with Dil-labeled macrophages and rituximab at the indicated concentrations. Phagocytosis after 2 hours (defined as CFSE+Dil+ singlet cells) was quantified by FC. Percent phagocytosis did not differ in the absence or presence of rituximab. (I) Similar to data in panel E, coculture of CART123 cells with alemtuzumab in the absence or presence of complement demonstrates CDC-mediated T-cell killing.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/17/10.1182_blood-2016-08-736041/4/m_blood736041f1.jpeg?Expires=1765921307&Signature=l7mqw45WAVUCs2TbNrJrbHmUZ9S2u8gSPYU06t28TNq5MHFWVRF~-eXLs54BU9r5rQHpN7DuT1pU8VMzxaTKgva~oyNtSD3VUr1eMkv6exZo4HxCV47B3xXVcezn3r1iu9pGuTjPgjy~P67t9z~PoEH7dALWCgx2L~h0Ja7~PVJl1u64FoTGtE~jVLnz0h2PJES40ydVxYx-uPyF61TczR~Yd6F74x6ny8-v4TayssDTG49AGciIUVSFY5MN~lVPGUdr~dSFRsVinRNH1hgHq0D8SmOpJrSaI~Ncvbm3ZkVZfx1YlzTMBrXtD3-e3aUaQeYLM50jKnc4D6e6Wujc1w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Serial ablation of CART123 with alemtuzumab in AML xenograft model. NSG mice were injected with luciferase-expressing MOLM14 cells at week 0 time point, then treated with saline, UTD, or CART123 (n = 10 mice per cohort) at week 1 time point. Some CART123-treated mice were subsequently treated with 1 dose of 1 mg/kg alemtuzumab (alem) IP at 1, 2, or 3 weeks following CART123 (denoted as colored bars or as “A”) (weeks 2, 3, or 4 time points; n = 5 to 8 mice per cohort). Animals were assessed by (A) BLI with (B) quantification of radiance as a surrogate for AML burden, then euthanized when moribund. Two CART123-treated animals (red line in panel A) were found dead at >3 months post-T cells without evidence of recurrent leukemia. Animals in CART123-induced “remission” without and with alemtuzumab were subsequently rechallenged with MOLM14. (C) AML burden by BLI and CD3-PacificBlue+ CART123 cells in peripheral blood were quantified during treatment. MOLM14 rechallenge of T-cell–ablated mice at the week 12 time point resulted in rapid AML progression and animal death versus sustained remission of rechallenged non–T-cell–ablated mice with CART123-induced AML remission (****P < .0001 by analysis of variance with log-rank test; denoted for alemtuzumab at week 3 [light green] or week 4 [blue] versus saline control [light blue], and for CART123 [red] versus saline at week 2 or week 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/17/10.1182_blood-2016-08-736041/4/m_blood736041f3.jpeg?Expires=1765921307&Signature=iL4-iNsbDWasxKqmSUYqcmN~unlDCZnAPWHOES8vQKneCFjWZhSNOmXOlN0qUidEYwobd01~F6OzOYGPTfGd7C22Wtu7g7Wk40xTmJubRNtmQfd3n6y8rNmGB-PefdeOuA6MLjJspUo~shCZuL9HMsIJMtWDIeC4F263NMoWobo2LVXPb0FPdK-Npu5pGJZETLHEMWyNhKOOq9Qmwo~dx8jLvMXgfH7Zk7SCxcYKGoFwJGNWxEr5JXfhVN~LfDrhj5o6ZH3c9jkmvDUmXYdB9~8SImYchmnLOlfQh8QVkdR2-B73F0J42Eu2GNNlYr9NJMN3apmE4EegrapkRw7bBA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. In vivo termination of CAR T cells with alemtuzumab in human AML xenograft models. (A) MOLM14-bearing NSG mice (n = 10 mice per cohort) were treated IV with saline (gray bar), 1 × 106 UTD (striped bar), or 1 × 106 CART123 (red bars) at week 1 time point and assessed by weekly BLI. CART123 induced AML remission by week 4. One dose of 1 mg/kg alemtuzumab was administered IP at 4 weeks following CART123 (week 5 time point; blue bar) in designated animals for T-cell depletion. MOLM14 rechallenge at week 9 resulted in rapid leukemia progression and animal death only in mice in which CART123 had been previously ablated with alemtuzumab. (B) Representative peripheral blood FC analysis of MOLM14-bearing, CART123-treated mouse before and after alemtuzumab (alem) administration (week 5 time point). No CD3-PacificBlue+ human T cells are detected at 24 hours (h) following a single dose of alemtuzumab. Blue gate denotes human T-cell count in murine blood normalized to quantitative counting beads. Note that fewer T cells remain in peripheral blood at this time point due to prior CART123-mediated AML clearance. (C) Immunohistochemical analysis of CD8+ T cells and CD33+ AML cells in harvested tissues of MOLM14-bearing mice treated with CART123 (week 2, 1 week after CART123) or CART123 with 1 mg/kg alemtuzumab (week 5, 24 hours after alemtuzumab/4 weeks after CART123). Note that animals euthanized at earlier time points in this subexperiment were not included in the main data analysis shown in panel A. (D) FC analyses of human CD45-APC+ CD33-PE+ CD123-PE-Cy7+ AML and CD3-PacificBlue+ CAR T cells in an AML PDX model (juvenile myelomonocytic leukemia [JMML] 117). Rapid T-cell depletion and sustained AML remission were observed in CART123-treated animals following a single dose of alemtuzumab (administered 24 hours prior to week 5 blood sampling). (E) End-study IHC analysis of murine bone marrow from saline and UTD control mice is hypocellular and fibrotic without evidence of residual AML, suggestive of advanced leukemia in these models. Marrow tissues from CART123-treated mice are more cellular and demonstrate AML clearance even after alemtuzumab administration.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/17/10.1182_blood-2016-08-736041/4/m_blood736041f4.jpeg?Expires=1765921307&Signature=fv4j29yZQTJWug~r8YiJ095wb61k90fOY9-KBhoNFvU6FdTjqA50OXtgAqwCbk9rQLhm9gj3RRCbNqJ6HYLNURFbYjjn0cZtjj2Y69TApBpnUPA5ufGEzkKHthTIRMMIhJ-dT2Ezq1DiXNGeXInb7ax-eqy~S9mFO6RrL-kVK-DnIr0uGRtOhSJM5ebD2OhM9l5wEJggsp~phjQzOyTXCTHDvUy1puTKHCLMZ8gDIcZFQ5wa2p95A8AT3vwbaXYkgtAqFBMrM44tCCDRrluEiH93vZQcyMryGk2spEUAsPaHaAzywW25SGhxOwc2yep5YCUJH07clpTpNxKpIY~z4g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Anti-AML efficacy of RNA-CART123 and of CART123/CD20 with rituximab depletion. (A) MOLM14-bearing NSG mice (n = 10 mice per cohort) were treated IV with saline, mock-RNA T cells × 3 doses, RNA-CART123 × 3 doses, CART123, or CART123-CD20 and assessed by BLI. Some mice were injected IP with 60 mg/kg cyclophosphamide diluted in 200 μL saline at 24 hours prior to second and third doses of mock-RNA T cells or RNA-CART123 to deplete previously administered T cells. RNA-CART123 and CART123-CD20 eradicated leukemia by 4 weeks post-T cells (week 5 time point), with similar kinetics to those of CART123, resulting in long-term animal survival. Ablation of CART123/CD20 with 1 mg/kg rituximab IP administered at week 5 did not alter leukemia remission status. MOLM14 rechallenge at week 9 resulted in rapid disease progression and animal death in mice previously treated with RNA-CART123, confirming lack of longer-term persistence of these T cells in vivo. MOLM14 rechallenge of CART123-CD20–treated animals subsequently treated with rituximab also resulted in rapid AML relapse, confirming successful prior ablation of CART123-CD20 in vivo. (B) Treatment of AML290-PDX mice (established as in Figure 4D; n = 8 mice per cohort) with 3 doses of RNA-CART123, as in panel A, induced human leukemia remission measured in murine tissues harvested at 6 weeks after the third dose of T cells (week 8 time point). (C) Treatment of juvenile myelomonocytic leukemia [JMML] 117 PDX mice with CART123 (123) or CART123-CD20 (123-CD20) at week 1 (n = 5 to 8 mice per cohort) resulted in marked reduction in CD45-APC+ CD33-PE+ CD123-PE-Cy7+ AML burden in murine tissues at 8 weeks post-T cells (week 9 time point). PDX mice treated with 1 dose of 1 mg/kg rituximab at the week 5 time point demonstrated sustained AML remission at the week 9 time point (8 weeks after CART123-CD20, 4 weeks after rituximab). (D) FC analysis of CD45-APC+ CD3-PacificBlue+ CAR T cells in peripheral blood of JMML117 PDX mice treated with CART123-CD20 without and with subsequent rituximab. Total CAR T-cell counts (blue gate) in murine blood normalized by quantitative counting beads are markedly reduced following rituximab treatment, although T-cell elimination is less rapid than with alemtuzumab treatment, as in Figure 3. Note that detectable CART123-CD20 decreased over time due to ongoing AML clearance from peripheral blood. (E) Immunohistochemical (IHC) staining of harvested murine spleen tissues, as in panel C, for CD33+ human AML and CD3+ CAR T cells at week 9 time point (8 weeks post-T cells) in JMML117 PDX model. Slides were scanned at ×20 magnification with an Aperio Scanscope CS-O slide scanner with visualization via the Aperio Image Analysis Toolkit (Leica Biosystems).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/129/17/10.1182_blood-2016-08-736041/4/m_blood736041f5.jpeg?Expires=1765921307&Signature=yb820Bf1OeWk8MjY~Oxfk1BPGtr2MQr43AmUIa6xUzPGPZonb71c8PHcXqgSZaVeW1BkGH2viHufgB7RiXeZpZlhoFL5uT5RQuSG-zzO43JZgr73YlE-jgCkeZXI80XtIBYSwbUaPAazLe4ZyzpPYxNSxve5lSW5frQBSy8y6liQq-XplQKeZ2fev5TCo03abE3AczwN-eUVZ7Nlb1~YrHJlSiaXnJUDbH3TvB82nD9Dr-6M9FEHMo2Q1oIEMKTHkxjGwQXa~ZEkVcScRL41-i1ernTyemXPCf6nNOtwvCx8JLmCPy4NAAyKnCBtZIQWvpHDlYPsjR6iyAfbdJwOCw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)