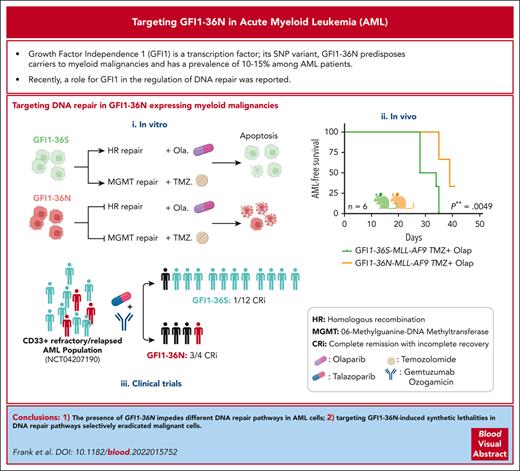
Presence of GFI1-36N impedes HR- and MGMT-mediated DNA repair selectively in AML cells.
Use of temozolomide and olaparib allows for selective targeting of GFI1-36N leukemic cells.
Visual Abstract
Growth factor independence 1 (GFI1) is a DNA-binding transcription factor and a key regulator of hematopoiesis. GFI1-36N is a germ line variant, causing a change of serine (S) to asparagine (N) at position 36. We previously reported that the GFI1-36N allele has a prevalence of 10% to 15% among patients with acute myeloid leukemia (AML) and 5% to 7% among healthy Caucasians and promotes the development of this disease. Using a multiomics approach, we show here that GFI1-36N expression is associated with increased frequencies of chromosomal aberrations, mutational burden, and mutational signatures in both murine and human AML and impedes homologous recombination (HR)–directed DNA repair in leukemic cells. GFI1-36N exhibits impaired binding to N-Myc downstream-regulated gene 1 (Ndrg1) regulatory elements, causing decreased NDRG1 levels, which leads to a reduction of O6-methylguanine-DNA-methyltransferase (MGMT) expression levels, as illustrated by both transcriptome and proteome analyses. Targeting MGMT via temozolomide, a DNA alkylating drug, and HR via olaparib, a poly-ADP ribose polymerase 1 inhibitor, caused synthetic lethality in human and murine AML samples expressing GFI1-36N, whereas the effects were insignificant in nonmalignant GFI1-36S or GFI1-36N cells. In addition, mice that received transplantation with GFI1-36N leukemic cells treated with a combination of temozolomide and olaparib had significantly longer AML-free survival than mice that received transplantation with GFI1-36S leukemic cells. This suggests that reduced MGMT expression leaves GFI1-36N leukemic cells particularly vulnerable to DNA damage initiating chemotherapeutics. Our data provide critical insights into novel options to treat patients with AML carrying the GFI1-36N variant.
Introduction
GFI1 transcriptionally regulates the development of hematopoietic, neuronal, and intestinal epithelial cells.1-5 A variant of GFI1 denominated GFI1-36N and characterized by an exchange of serine (S) to asparagine (N) at position 36 has a prevalence of 5% to 7% in different healthy control populations. The prevalence of the GFI1-36N allele is increased (10%-15%) among patients with MDS, acute myeloid leukemia (AML), and multiple myeloma, and the presence of the GFI1-36N allele is associated with a poor prognosis.6-8GFI1-36N leukemic cells feature increased H3K9 acetylation at target genes, resulting in higher expression of genes such as Hoxa9, Pbx1, Meis1, CSF1, and CSFR1,9 driving cell survival and proliferation.10-16 GFI1 also regulates apoptosis through regulating the methylation status of p53 in lymphoblastic leukemia17 and MRE11 and 53BP1 in DNA repair.18 However, it is not known how these nontranscriptional activities are affected in the GFI1-36N variant.
We leveraged multiomics profiling to gain mechanistic insights into the molecular architecture that drives myeloid leukemia in the presence of GFI1-36N. GFI1-36N interferes with DNA repair in leukemic cells and sensitizes malignant cells to treatment with olaparib and temozolomide, opening a new therapeutic approach to treat AML/MDS.
Materials and methods
Patient cohort
Patients with AML treated in Essen, Hannover, and Dresden (Study Alliance Leukemia AML registry biobank [IRB no. EK98032010]) as well as the MLL cohort were described previously.6,19-22 All experiments with human samples were carried out in accordance with the approved protocol of the respective competent authority. All the patients provided written informed consent and performed according to the Declaration of Helsinki.
Mouse strains and approval
Mice carrying either the GFI1-36N or GFI1-36S allele were generated as described previously.17 NUP98-HOXD13 transgenic mice were obtained from The Jackson Laboratory (Bar Harbor, ME).9,22 PiggyBac Transposon mouse models were donated by the Trust Sanger Institute, Hinxton-Cambridge.23,24 All mice were kept under specific-pathogen-free conditions, and all animal experiments were approved by the respective animal ethics committee (North Rhine-Westphalia: 84-02.04.2015.A076, 81-02.04.2019.A440 or Regierungsbezirk Oberbayern: 55.2Vet-2532.Vet_03-16-56).
Generation of leukemic cells
Lineage-negative (Lin–) cells were isolated from the total bone marrow (BM) of knockin mice carrying either human GFI1-36N or human GFI1-36S using the Lineage Cell Depletion Kit (Miltenyi Biotec, catalog no. 130-090-858 and catalog no. 130-042-401). Lin– cells were then cultured in IMDM media containing 20% fetal bovine serum, 1% penicillin/streptomycyn, 10 ng/ml murine interleukin-3 (mIL-3), 10 ng/mL mIL-6, and 20 ng/ml murine stem cell factor (Miltenyi Biotec, catalog no. 130-101-741, catalog no. 130-096-687, and catalog no. 130-094-065) for expansion. Lin– cells were then transduced retrovirally with the MSCV (murine stem cell virus)-MLL (mixed lineage leukemia)-AF9-IRES (internal ribosomal entry site)–green fluorescent protein (GFP) (MLL-AF9; kindly provided by Jay Hess) plasmid9,25 expressing GFP. A total of 1 × 105 positive transduced (GFP+) Lin− cells were transplanted IV together with 1 × 105 competitive BM cells into lethal (7 + 3 Gy)-irradiated C57BL/6 mice. Leukemic BM cells corresponding to 1 × 105 GFP+ cells were retransplanted IV into sublethal irradiated C57BL/6 mice (3 Gy), as previously described.9,25
Label-free proteome quantification
Fluorescence activated cell–sorted GFI1-36N or GFI1-36S MLL-AF9–expressing leukemic cells (0.5-1 × 106 cells per sample) from the mouse BM were thoroughly washed in plain phosphate buffered saline (PBS), lysed in 1% sodium deoxycholate (SDC) buffer (1% SDC, 100 mM Tris pH8.5, 40 mM 2-chloroacetamide, and 10 mM tris(2-carboxyethyl)phosphine), incubated on ice for 20 minutes, boiled at 95°C, and sonicated for 5 minutes on a Biorupter plus, as described previously.26 Samples were digested with the proteases LysC (1:100 ratio) for 2 hours, followed by trypsin (1:100 ratio) overnight at 37°C. To the digested peptide volume, 5 times the volume of isopropanol/1% trifluoroacetic acid (TFA) was added and vortexed to stop the digestion. The peptides were desalted on equilibrated styrenedivinylbenzene-reversed phase sulfonated StageTips, washed once in isopropanol/1% TFA and twice with 0.2% TFA. Purified peptides were eluted with 60 μL of elution buffer (80% acetonitrile and 1.25% NH4OH). The dried elutes were resuspended in MS loading buffer (3% ACN, 0.3% TFA) and stored at −20°C until MS measurement. For liquid chromatography mass spectrometry (LC-MS/MS) measurement, we used 200 ng peptide (concentration determined by nanodrop) per sample. Further details can be found in supplemental Methods, available on the Blood website. For samples from patients with AML, mononuclear cells were ficoll-enriched, washed twice in PBS, lysed, and processed as mentioned earlier.
In vitro and in vivo treatment of primary cells with temozolomide and olaparib
Temozolomide (Sigma) and olaparib (Selleckchem or MedChemExpress or AstraZeneca) were dissolved in dimethyl sulfoxide (39 mg/ml and 86 mg/mL, respectively). For the colony-forming unit assays, 0.5 × 103 to 1 × 104 primary murine cells were plated in 1 mL MethoCult media (M3434, Stemcell) and 0.5 × 103 primary human AML cells were plated in 1 mL MethoCult media (H4434, Stemcell) in 6-well plates, and the colonies were counted after 10 days of incubation. To measure the cell viability, metabolic activity was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Abcam), which was performed according to the manufacturer's protocol. For the MTT assay, cells were plated at a density of 3 × 105 cells per mL, and temozolomide was added in a range between 5 μg/mL and 400 μg/mL and incubated at 37°C and 5% CO2 for 48 hours. After the drug treatment, the media was replaced with MTT reagent and incubated for 3 hours, followed by MTT solvent for 15 minutes. The absorption was measured at 590 nm using a Victor X3 Multimode Plate Reader (Perkin Elmer). For in vivo drug treatments, working solutions of 10 mg/mL temozolomide and 20 mg/mL olaparib were prepared freshly on the day of treatment with PBS. Mice were treated intraperitoneally according to their weight with olaparib and with temozolomide (temozolomide 50 mg/kg [days 2-4] and olaparib 100 mg/kg [days 2-3]). The procedure was repeated if mice were deemed suitable according to their status and scoring.
Statistical analysis
GraphPad Prism 6 was used for the statistical analyses. Significance was calculated using paired or unpaired two-sided t tests.
Clinical trial NCT04207190
This phase 1/1b trial studies the safety profile and potential efficacy of talazoparib in combination with gemtuzumab ozogamicinin CD33-positive patients with AML who are relapsed or refractory. Patient samples were genotyped as previously described.6
Routine protocols and procedures are described in detail in supplemental Methods.
Results
Presence of GFI1-36N is associated with increased DNA damage and compromised DNA repair
The presence of a GFI1-36N allele was associated with an increased frequency of chromosomal aberrations in 3 cohorts of patients with AML (Essen, Dresden, and Hannover) independent of age, sex, and French American British (FAB) classification (Figure 1A-B; supplemental Tables 1 and 2). The allele did not correlate with a particular molecular alteration, confirming our previous observations for patients with MDS.7 The demographic details, FAB type, blood analysis, and mutational status of the Hannover patient cohort are shown in supplemental Tables 3 to 8. To gain further molecular insight, we used GFI1-36S and GFI1-36N knockin mice.9,17 We generated GFI1-36S or GFI1-36N myeloid leukemia cells expressing the MLL-AF9 oncofusion protein25 and performed 4 rounds of serial transplantation to allow sufficient time for the effects of reduced DNA repair capacity to become detectable (Figure 1C). The frequency of deletions, insertions, and mutations was significantly higher in GFI1-36N than those in GFI1-36S leukemic cells (Figure 1D). Furthermore, GFI1-36N leukemic cells showed more missense mutations than GFI1-36S leukemic cells (Figure 1E), but the frequency of large-scale chromosomal aberrations remained the same in both types of leukemic cells (supplemental Figure 1A). However, microdeletions (detected by array–comparative genomic hybridization) were observed more frequently in GFI1-36N leukemic cells, with 3.33 ± 0.33 more microdeletions than GFI1-36S–expressing leukemic cells (supplemental Figure 1B) but did not reach significance, likely because of the small sample size. To confirm our results, we used an additional murine AML model that causes insertional mutagenesis specifically in hematopoietic cells by means of a transposon-transposase system (Figure 1F),23 allowing for the targeted sequencing of the genomic areas to which the transposon has been relocated. The presence of GFI1-36N was associated with a significantly increased number of common insertion sites of the transposon compared to GFI1-36S–expressing cells (Figure 1G). GFI1-36N–associated insertion sites were frequently found proximal to genes involved in DNA repair such as TRIM44, MECOM, and ZEB2 (supplemental Figure 2A). The presence of GFI1-36N correlated with clonal aberrant karyotypes, yet the overall sample number was too low to gain statistical significance (supplemental Figure 2B).
More genetic aberrations in human and murine GFI1-36N AML samples. (A) Percentage of patients with MDS/AML of 3 different cohorts with >2 chromosomal aberrations. Patient samples were genotyped for the presence of GFI1-36N or GFI1-36S with real time (RT)-PCR. (B) Number of patients in individual cohorts corelating to gender and age of the patients. (C) Schematic experimental setup to generate leukemic mice and the serial transplantation experiments. (D) Serial transplanted BM cells from leukemic MLL-AF9 mice and nonleukemic Lin– cells were analyzed using RNA-seq followed by variant calling analysis. Shown is the number of variations in leukemic cells minus the number of variations in nonleukemic cells. n = 3; mean ± standard deviation. (E) Variations from (D) divided according to the functional class of mutation. Shown is the total number of mutations per genotype (left). The Venn diagram (right) represents the overlaps of missense mutations between GFI1-36S and GFI1-36N leukemic cells. (F) Scheme of the PiggyBac transposon-based mouse model. GFI1-36S or GFI1-36N mice were crossed with the PiggyBac transposon mice (Mx-Cre × Rosa26 × ATP2). Mice were injected with poly(I:C) to activate the transposon system. (G) The PiggyBac transposon-based mouse model was used to check the number of common insertion sites (CISs) of the transposon sequence. The number of CISs were calculated for each genotype. WT: n = 4, GFI1-36S (heterozygous [n = 6] and homozygous [n = 1]): n = 7, and GFI1-36N (heterozygous [n = 2] and homozygous [n = 7]): n = 9. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. M, men; W, women; WT, wild-type.
More genetic aberrations in human and murine GFI1-36N AML samples. (A) Percentage of patients with MDS/AML of 3 different cohorts with >2 chromosomal aberrations. Patient samples were genotyped for the presence of GFI1-36N or GFI1-36S with real time (RT)-PCR. (B) Number of patients in individual cohorts corelating to gender and age of the patients. (C) Schematic experimental setup to generate leukemic mice and the serial transplantation experiments. (D) Serial transplanted BM cells from leukemic MLL-AF9 mice and nonleukemic Lin– cells were analyzed using RNA-seq followed by variant calling analysis. Shown is the number of variations in leukemic cells minus the number of variations in nonleukemic cells. n = 3; mean ± standard deviation. (E) Variations from (D) divided according to the functional class of mutation. Shown is the total number of mutations per genotype (left). The Venn diagram (right) represents the overlaps of missense mutations between GFI1-36S and GFI1-36N leukemic cells. (F) Scheme of the PiggyBac transposon-based mouse model. GFI1-36S or GFI1-36N mice were crossed with the PiggyBac transposon mice (Mx-Cre × Rosa26 × ATP2). Mice were injected with poly(I:C) to activate the transposon system. (G) The PiggyBac transposon-based mouse model was used to check the number of common insertion sites (CISs) of the transposon sequence. The number of CISs were calculated for each genotype. WT: n = 4, GFI1-36S (heterozygous [n = 6] and homozygous [n = 1]): n = 7, and GFI1-36N (heterozygous [n = 2] and homozygous [n = 7]): n = 9. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. M, men; W, women; WT, wild-type.
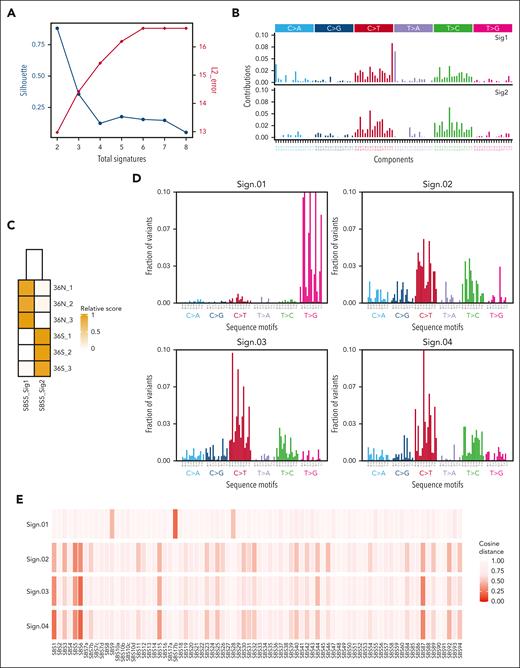
We determined the mutational signature of murine GFI1-36S and GFI1-36N leukemic cells from our MLL-AF9 model, performing analyses on synonymous and nonsynonymous variants and identifying 2 different clusters (k = 2; Figure 2A). Matching the corresponding signatures to Catalogue of Somatic Mutations in Cancer (COSMIC) signatures,27 both signatures (Sig1 and Sig2) were similar to single-base substitution 5 (SBS5; similarity > 0.70), which is potentially associated with mutational processes related to aging and nucleotide excision repair. However, each mouse line (GFI1-36N and -36S) was matched to a different subtype of SBS5 (Figure 2C), mainly driven by different adjacent bases (5′ and 3′ of the mutation) of C>T and T>C mutations (Figure 2B).
Somatic signatures in GFI1-36S and -36N leukemic mice and de novo identification of mutational signatures in human GFI1-36N–mutated samples. (A-C) RNA-seq data of BM cells from leukemic GFI1-36S (n = 3) and GFI1-36S (n = 3) mice were analyzed regarding their somatic signatures. (A) The optimal number of signatures is estimated based on silhouette coefficient (black) and L2 error (red). (B) SBS profiles considering the mutated base but also the bases immediately 5′ and 3′ for each signature and (C) signature activities for each sample. (D-E) Human GFI1-36S (n = 1348) and GFI1-36N (n = 182) samples were analyzed for their mutational signatures. (D) Mutational signatures identified in somatically enriched set of variants occurring in coding regions of clinical samples harboring GFI1-36N. (E) Cosine similarity of signatures Sign.01 to Sign.04 to the COSMIC single-base substitution reference set of mutational signatures version 3.3.
Somatic signatures in GFI1-36S and -36N leukemic mice and de novo identification of mutational signatures in human GFI1-36N–mutated samples. (A-C) RNA-seq data of BM cells from leukemic GFI1-36S (n = 3) and GFI1-36S (n = 3) mice were analyzed regarding their somatic signatures. (A) The optimal number of signatures is estimated based on silhouette coefficient (black) and L2 error (red). (B) SBS profiles considering the mutated base but also the bases immediately 5′ and 3′ for each signature and (C) signature activities for each sample. (D-E) Human GFI1-36S (n = 1348) and GFI1-36N (n = 182) samples were analyzed for their mutational signatures. (D) Mutational signatures identified in somatically enriched set of variants occurring in coding regions of clinical samples harboring GFI1-36N. (E) Cosine similarity of signatures Sign.01 to Sign.04 to the COSMIC single-base substitution reference set of mutational signatures version 3.3.
To test whether these results can be recapitulated in humans, a cohort of 1530 patients diagnosed with AML or MDS with 182 carriers of the GFI1-36N variant was investigated. Using de novo mutational signature identification for these 182 patients, we identified 4 different signatures (Figure 2D; supplemental Figure 3A-B). Sign.01 likely has a technical origin, because we see it consistently in other cohorts as well. However, Sign.02, Sign.03, and Sign.04 showed similarity to COSMIC reference signatures SBS6, SBS5, SBS1, and SBS87 (Figure 2E) and were similar to Sig1 and Sig2 observed in murine samples (Figure 2B). A comparison of the murine and human results indicated that there are signatures in both data sets that are based on C>T mutations and, to a lesser extent, on T>C (Figure 2B,D).
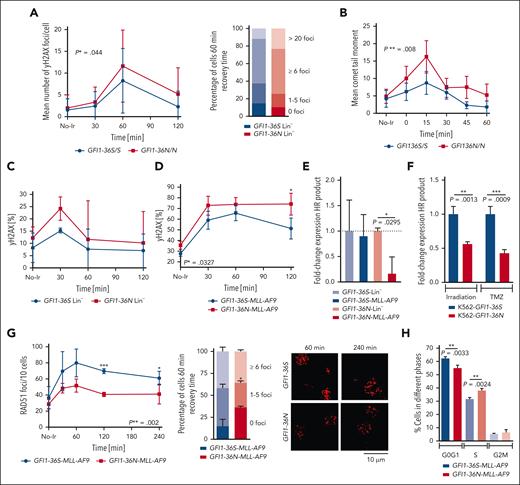
To further investigate global DNA damage response and repair, we first used murine thymocytes, an immune cell type expressing higher levels of GFI1 than BM cells, and tested their response to DNA damage with comet assays and by measuring γH2AX foci after exposure to irradiation. An 80% increased tail moment and a 40% higher number of γH2AX foci (both P ≤ .05) were observed in GFI1-36N thymocytes than in GFI1-36S cells (Figure 3A-B). However, the slope of the decreasing number of foci and tail moment during the repair phase was similar between GFI1-36N and GFI1-36S–expressing cells. In addition, GFI1-36N Lin– cells from the mouse BM showed a higher number of γH2AX foci than GFI1-36S control cells, but both cell types had a similar rate of DNA repair slope (Figure 3C). Although leukemic GFI1-36N cells also demonstrated higher DNA damage upon exposure to the same dose of irradiation compared with leukemic GFI1-36S control cells, they showed a reduced DNA repair capacity compared with GFI1-36S leukemic cells (Figure 3D).
Higher DNA damage in GFI1-36N cells and lower DNA repair in GFI1-36N leukemic cells. (A) γH2AX-assay results of GFI1-36S and GFI1-36N thymocytes. γH2AX foci were stained with an antibody against γH2AX (immunofluorescence staining) and counted at different time points after 2 Gy irradiation. The images were analyzed with Imaris. Approximately 50-176 cells per sample; mean ± standard deviation (SD). P value was calculated over time. (B) Alkaline comet assay results of GFI1-36S and GFI1-36N thymocytes at different time points after irradiation with 5 Gy. Tail moment was analyzed from 34-65 cells per sample with the Comet-Assay Software from CaspLab. Mean ± SD. P value was calculated over time. (C) Murine nonleukemic progenitor cells (Lin– cells) or (D) leukemic (MLL-AF9) BM cells from mice that received transplantation were irradiated with 3 Gy and were analyzed by flow cytometry at different time points after irradiation γH2AX level. n = 3; mean ± SD. (E) HR assay results from murine GFI1-36S- (n = 2) and GFI1-36N- (n = 2) Lin– cells and murine GFI1-36S- (n = 2) and GFI1-36N- (n = 2) MLL-AF9 BM cells. The HR rate was measured with RT-PCR after the cells were transfected with 2 plasmids of the plasmid-based homologous recombination assay from Norgen Biotek Corp; mean ± SD (F) K562 cell lines expressing GFI1-36S or GFI1-36N were generated by CRISPR/Cas. The HR rate was measured as described in (E) 2 hours after irradiation with 3 Gy or 24 hours after treatment with 100 μg/mL temozolomide (TMZ). N = 3, mean ± SD (G) RAD51 foci formation in murine GFI1-36S and GFI1-36N leukemic (MLL-AF9) BM cells was analyzed at different time points after irradiation with 5 Gy. n = 3 (31-73 cells per sample), mean ± SD. ∗P > .05; ∗∗P < .01; ∗∗∗P < .001. (H) Cell-cycle analysis of GFI1-36S and GFI1-36N MLL-AF9 cells. GFI1-36S: n = 3 and GFI1-36N: n = 4; mean ± SD. ∗∗P < .01. No-Ir: no irradiation (0 Gy).
Higher DNA damage in GFI1-36N cells and lower DNA repair in GFI1-36N leukemic cells. (A) γH2AX-assay results of GFI1-36S and GFI1-36N thymocytes. γH2AX foci were stained with an antibody against γH2AX (immunofluorescence staining) and counted at different time points after 2 Gy irradiation. The images were analyzed with Imaris. Approximately 50-176 cells per sample; mean ± standard deviation (SD). P value was calculated over time. (B) Alkaline comet assay results of GFI1-36S and GFI1-36N thymocytes at different time points after irradiation with 5 Gy. Tail moment was analyzed from 34-65 cells per sample with the Comet-Assay Software from CaspLab. Mean ± SD. P value was calculated over time. (C) Murine nonleukemic progenitor cells (Lin– cells) or (D) leukemic (MLL-AF9) BM cells from mice that received transplantation were irradiated with 3 Gy and were analyzed by flow cytometry at different time points after irradiation γH2AX level. n = 3; mean ± SD. (E) HR assay results from murine GFI1-36S- (n = 2) and GFI1-36N- (n = 2) Lin– cells and murine GFI1-36S- (n = 2) and GFI1-36N- (n = 2) MLL-AF9 BM cells. The HR rate was measured with RT-PCR after the cells were transfected with 2 plasmids of the plasmid-based homologous recombination assay from Norgen Biotek Corp; mean ± SD (F) K562 cell lines expressing GFI1-36S or GFI1-36N were generated by CRISPR/Cas. The HR rate was measured as described in (E) 2 hours after irradiation with 3 Gy or 24 hours after treatment with 100 μg/mL temozolomide (TMZ). N = 3, mean ± SD (G) RAD51 foci formation in murine GFI1-36S and GFI1-36N leukemic (MLL-AF9) BM cells was analyzed at different time points after irradiation with 5 Gy. n = 3 (31-73 cells per sample), mean ± SD. ∗P > .05; ∗∗P < .01; ∗∗∗P < .001. (H) Cell-cycle analysis of GFI1-36S and GFI1-36N MLL-AF9 cells. GFI1-36S: n = 3 and GFI1-36N: n = 4; mean ± SD. ∗∗P < .01. No-Ir: no irradiation (0 Gy).
We first performed an assay to follow homologous recombination (HR) with a polymerase chain reaction (PCR) approach.28 Murine GFI1-36N leukemic cells had a 75% diminished capacity for HR compared with GFI1-36N nonleukemic Lin– progenitor cells, GFI1-36S MLL-AF9 leukemic cells, or GFI1-36S nonleukemic Lin– progenitor cells (Figure 3E). To confirm this observation in an independent human leukemia model, K562 cell lines with 1 GFI1-36N allele were generated using CRISPR/Cas. Upon irradiation or treatment with the alkylans temozolomide, K562 cells with a GFI1-36N allele showed reduced HR repair capacity compared with cells carrying both GFI1-36S alleles (Figure 3F). This diminished rate of HR in the murine or human models was not due to reduced expression of RAD51 (a key HR facilitator)29-31 at steady state (supplemental Figure 3C). To check whether a potentially different binding between GFI1-36S and GFI1-36N with RAD51 could explain the detected impeded HR and the different RAD51 foci formation in GFI1-36N leukemic cells, we performed immunofluorescence experiments, and we did not observe a different colocalization of GFI1-36S or GFI1-36N with RAD51 (supplemental Figure 3D). However, the number of RAD51 foci appearing after 5 Gy irradiation was significantly reduced in GFI1-36N leukemic cells compared with that in GFI1-36S leukemic cells, confirming the results obtained with the plasmid-based HR assay (Figure 3G).
53BP1 regulates the double-stranded break repair pathway choice between HR and nonhomologous end joining (NHEJ) by promoting the NHEJ S phase.31 We probed the formation of 53BP1 foci but did not find significant differences between GFI1-36S and GFI1-36N leukemic cells (supplemental Figure 3E) nor a colocalization between GFI1 and 53BP1 (supplemental Figure 3F), indicating that the capacity for NHEJ remained unchanged in the presence of the variant GFI1 allele. Coordinated DNA repair depends on proper control of cell-cycle status, and HR-directed DNA repair occurs mostly in G2 or S phases. The observed reduction of HR-directed DNA repair in the presence of a GFI1-36N allele was not due to a reduction of cells in the S or G2/M phases. On the contrary, a higher proportion of GFI1-36N leukemic cells was in S phase compared with GFI1-36S leukemic cells (Figure 3H), suggesting a direct involvement of the GFI1 variant of the DNA repair machinery.
Presence of GFI1-36N deregulates MGMT levels
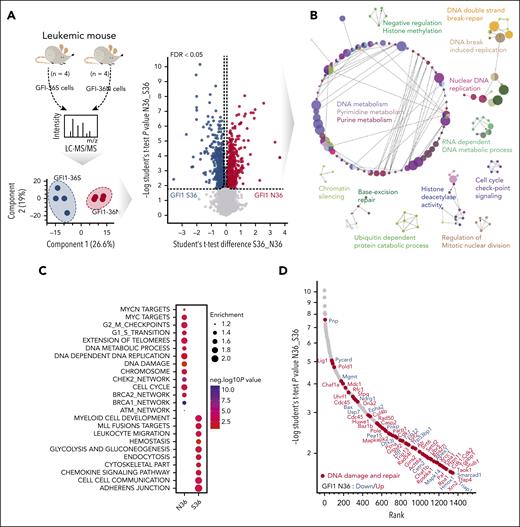
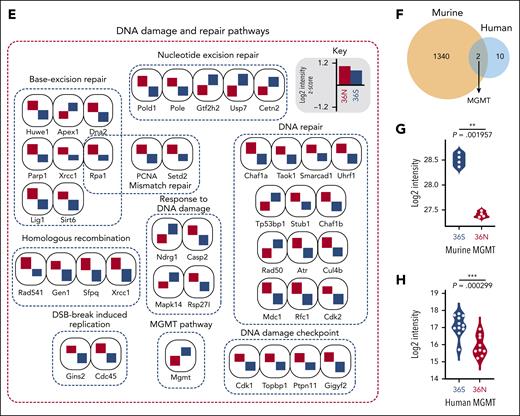
Using in-depth quantitative proteome analysis of murine GFI1-36S and GFI1-36N leukemic cells (n = 4, biological replicates. Figure 4A), we quantified nearly 7000 proteins at a peptide and protein false discovery rate of 1%, of which we found 1353 proteins to be differentially regulated (permutation-based false discovery rate [FDR] < 0.05; Figure 4A; supplemental Data 1) between the genotypes. We then performed biological process and pathway enrichment analysis using Cytoscape. DNA metabolism, DNA double-strand break and repair, DNA damage and repair pathway, base excision repair pathway, chromatin silencing, and cell-cycle checkpoint signaling (Figure 4B) were among these pathways, and this was in congruence with the functional data presented in Figure 3 regarding these processes. We found DNA damage, cell-cycle, cellular Myc (c-Myc) targets, CHEK2 network, ataxia-telangiectasia mutated (ATM) network, BRCA1 and -2 network specifically enriched in a GFI1-36N–dependent fashion (Figure 4C), suggesting that GFI1-36N cells undergo extensive DNA damage and repair. We focused then on DNA damage and repair pathways and could identify 66 proteins that were either upregulated or downregulated depending on the presence of GFI1-36N (Figure 4D, permutation-based FDR < 0.05). Most of the DNA damage-sensor proteins were upregulated in GFI1-36N leukemic cells (Figure 4E), supporting the notion that these cells experience more DNA damage than GFI1-36S leukemic cells.
Proteome discovers GFI1-36N leukemic cells deregulate DNA repair protein MGMT. Proteomic analysis of primary murine and human GFI1-36S and GFI1-36N leukemic cells (A) Schematic representation of the experimental setup of murine GFI1-36S (n = 4) vs GFI1-36N (n = 4) leukemic BM cells proteome experiment, principle component analysis of measured samples, and evaluation of the results (left). Volcano plot displays significantly regulated proteins (permutation-based FDR < 0.05) is shown (right). (B) Network analysis of significantly regulated proteins between the 2 genotypes using Cytoscape. The network displays enriched Gene Ontology Biological Processes terms (P < .01). (C) Dot plot showing the significantly enriched GFI1-36N and GFI1-36S specific gene set enrichment terms based on proteomic analysis (D) Rank plot displaying the total number of DNA damage and repair proteins (highlighted with red dots) of all significantly regulated proteins in the data set. (E) Significantly regulated DNA damage and repair proteins grouped according to their specific pathways (selected from panel D). Rank plot displaying all significantly regulated proteins in GFI1 36N and 36S leukemia cells. The protein that participates in DNA damage repair are highlighted in red dots. The GFI1 36N upregulated proteins are marked in red, and downregulated proteins are marked in blue. The x-axis denotes the protein rank and y-axis denotes the –log10 P value. (F) Venn diagram showing the unique and shared number of differentially expressed proteins in murine and human AML samples (also see supplemental Figure 4H). MGMT is 1 of the shared and significantly altered proteins in both mouse and human samples. (G) Violin plot showing the log2 intensity of MGMT protein level in primary GFI1-36S (n = 4) vs GFI1-36N (n = 4) murine leukemic BM cells. (H) Violin plot showing the log2 intensity of MGMT protein level in human GFI1-36S (n = 11) vs GFI1-36N (n = 9) cells from patients with AML patient.
Proteome discovers GFI1-36N leukemic cells deregulate DNA repair protein MGMT. Proteomic analysis of primary murine and human GFI1-36S and GFI1-36N leukemic cells (A) Schematic representation of the experimental setup of murine GFI1-36S (n = 4) vs GFI1-36N (n = 4) leukemic BM cells proteome experiment, principle component analysis of measured samples, and evaluation of the results (left). Volcano plot displays significantly regulated proteins (permutation-based FDR < 0.05) is shown (right). (B) Network analysis of significantly regulated proteins between the 2 genotypes using Cytoscape. The network displays enriched Gene Ontology Biological Processes terms (P < .01). (C) Dot plot showing the significantly enriched GFI1-36N and GFI1-36S specific gene set enrichment terms based on proteomic analysis (D) Rank plot displaying the total number of DNA damage and repair proteins (highlighted with red dots) of all significantly regulated proteins in the data set. (E) Significantly regulated DNA damage and repair proteins grouped according to their specific pathways (selected from panel D). Rank plot displaying all significantly regulated proteins in GFI1 36N and 36S leukemia cells. The protein that participates in DNA damage repair are highlighted in red dots. The GFI1 36N upregulated proteins are marked in red, and downregulated proteins are marked in blue. The x-axis denotes the protein rank and y-axis denotes the –log10 P value. (F) Venn diagram showing the unique and shared number of differentially expressed proteins in murine and human AML samples (also see supplemental Figure 4H). MGMT is 1 of the shared and significantly altered proteins in both mouse and human samples. (G) Violin plot showing the log2 intensity of MGMT protein level in primary GFI1-36S (n = 4) vs GFI1-36N (n = 4) murine leukemic BM cells. (H) Violin plot showing the log2 intensity of MGMT protein level in human GFI1-36S (n = 11) vs GFI1-36N (n = 9) cells from patients with AML patient.
To explore whether these changes might be explained by altered gene expression, we first performed RT2 profiler PCR arrays for DNA repair genes and observed that 40 out of 84 genes were differentially expressed (supplemental Table 9). We further confirmed this by bulk transcriptome analysis on BM cells from leukemic GFI1-36S and GFI1-36N mice (MLL-AF9 model). A gene set enrichment analysis revealed differentially expressed genes belonging to the DNA repair and p53 pathways and genes such as Gen1, Cul4b, and Dna (supplemental Figure 4A-C), although not all genes deregulated in the RT2 approach were deregulated in the RNA sequencing (RNA-seq) approach and vice versa.
The enzyme O6-methylguanine-DNA-methyltransferase (MGMT) catalyzes the transfer of methyl groups from O(6)-alkylguanine in the DNA to itself, thereby repairing these lesions.32 MGMT was among the most downregulated protein and transcript levels in GFI1-36N leukemic cells compared with that in GFI1-36S leukemic cells (Figure 4D-E; supplemental Figure 4D-F). The Mgmt RNA and protein expression remained unchanged in nonleukemic progenitor cells expressing GFI1-36S or -36N (supplemental Figure 4G-H), suggesting that the leukemic condition contributed to the reduced expression of Mgmt and Mgmt. To further explore this, we performed a deep proteome analysis of samples of patients with AML (genotypes GFI1-36S [n = 11] and GFI1-36N [n = 9]). The details of the patient samples used for proteomics analysis are listed in supplemental Tables 10 and 11). We profiled 165 patients with AML to identify 9 samples as GFI1-36N heterozygous carriers. The samples were selected evenly across disease stages and cytogenetic groups to counteract a potential subtype-specific bias. From a total of 8027 proteins identified, 6058 proteins were quantified in 70% of at least 1 genotype; significant analysis found 12 proteins were differentially regulated (permutation-based FDR < 0.05; details in supplemental Methods) between the 2 genotypes (supplemental Figure 4I; supplemental Data 2), and these include poly-ADP ribose polymerase (PARP) 14, KIN, HAUS5, and RAD18, involved in DNA damage, genome stability, and the DNA-binding protein PAPD5. Of these, 2 proteins were shared between murine and human samples (Figure 4F). Both murine and human AML samples showed downregulated Mgmt/MGMT levels in leukemic GFI1-36N cells compared with GFI-36S cells (Figure 4G-H). This strongly suggests that the expression of MGMT is reduced in AML cells expressing GFI1-36N, both at the RNA and protein levels. We further confirmed our findings in a published AML proteome data set of 177 samples.33 The GFI1-36N were identified by single nucleotide variant calling using the RNA-seq data (supplemental Figure 4J) and compared those for all GFI1-36S samples or the top 10 most GFI1-36S–expressing samples for equal variance comparison (supplemental Figure 4K-L).
Of note, we assessed the 66 DNA damage repair proteins and their transcript regulation systematically by correlation analysis. In general, the overall and genotype-specific sample transcriptome-to-proteome correlation was Pearson R = 0.46 to 0.48 (supplemental Figure 5A-C; supplemental Data 3), similar to that of our previous work.34 We performed comparative analysis to find shared and unique genes between the data sets (supplemental Figure 5D). The gene ontology enrichment analysis identified genes changing in both RNA proteins that are enriched for protein binding, translation, ribosomal RNA processing, ribosome subunits, DNA break and repair pathway, and caspase complex (supplemental Figure 5E). In the transcript only, upregulated genes enrich for terms such as protein binding, ribonucleo protein complex, noncoding RNA processing, messenger RNA processing, and splicing (supplemental Figure 5F). Genes upregulated at the protein but not at the transcript level were enriched for response to stress, translation, DNA replication, DNA repair proteins, the MCM complex, histone modifications, and catalytic activity (supplemental Figure 5G). This analysis points out that the DNA break and repair pathways are subjected to changes, as reflected in both RNA-protein levels in our data set. A direct transcriptome-proteome correlation suggests MGMT might not be regulated by posttranscriptional regulation (supplemental Figure 5H). However, when looking at the 66 selected DNA damage repair proteins in both the overall and genotype-specific transcriptome-to-proteome correlation, the data suggest some of these genes might undergo posttranscriptional regulation (supplemental Figure 5I-K; supplemental Data 3) because their transcript abundance correlates inversely to protein.
NDRG1 regulates MGMT levels in GFI1-36N AML cells
We next investigated why the presence of GFI1-36N in leukemic cells leads to lower levels of Mgmt/ MGMT. An altered methylation status of the MGMT promoter was not the reason (supplemental Figure 6A-C),35,36 nor does GFI1 bind to regulatory elements of the MGMT locus, based on published chromatin immunoprecipitation (ChIP)-seq data sets from CODEX (GSE31657, GSE69101, GSE50806, and GSE42518).17,33,37,38 The presence of GFI1-36N also had no effect on the RNA stability of Mgmt of murine GFI1-36S and -36N leukemic cells (Figure 5A). The GFI1 interactome (using mass spectrometry [AP-MS]) revealed known interactors, such as Histon deacetylase and protein-arginine methyltransferase 1 but did not reveal a direct interaction of GFI1 with MGMT (supplemental Figure 6D-F).
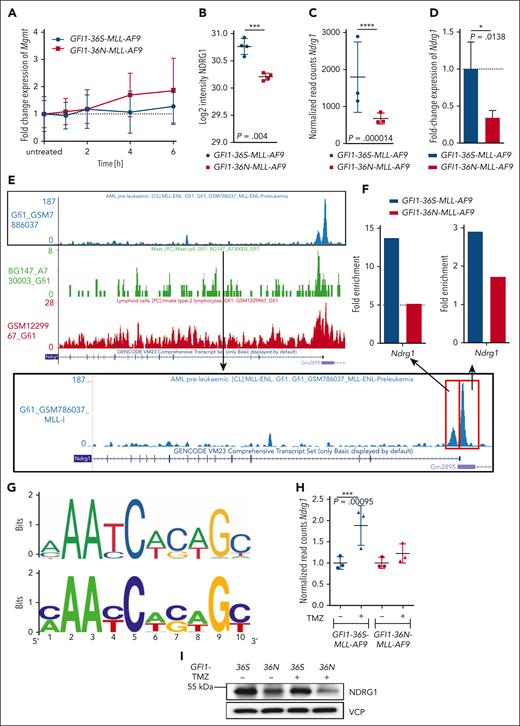
MGMT downregulation in GFI1-36N cells due to low levels of NDRG1. (A) Fold change expression of Mgmt in murine GFI1-36S- and GFI1-36N-MLL-AF9 leukemic BM cells at different time points after actinomycin D (10 μg/mL) treatment. Mgmt level was normalized to Hprt and to the untreated controls. n = 3; mean ± SD. (B) NDRG1 protein level in GFI1-36S- and GFI1-36N-MLL-AF9 leukemic BM cells (proteomic). n = 4; mean ± SD. (C) Ndrg1 expression (normalized read counts; RNA-seq) of murine leukemic GFI1-36S- and GFI1-36N-MLL-AF9 BM cells. n = 3; mean ± SD. (D) Ndrg1 gene expression measured in GFI1-36S- and GFI1-36N-MLL-AF9 BM cells by RT-PCR. GFI1-36S: n = 3 and GFI1-36N: n = 3; mean ± SD. (E) Published GFI1-ChIP-seq data sets showing the Ndrg1 gene and its regulatory elements with the possible binding sides of GFI1 (red square) at regulatory elements of Ndrg1. (F) GFI1-ChIP-quantitative PCR of the Ndrg1 upper regulatory elements of murine GFI1-36S and GFI1-36N leukemic BM cells. Gapdh and Runx1 were used as a control (right). (G) Comparison between the GFI1 binding motif from the Jasper database (top) and the consensus motif found using find individual motif occurrence (FIMO) at sites occupied by GFI1 in 21 genes differentially expressed in granulocyte/monocyte progenitors s from GFI1-36N or -36S animals. (H) Ndrg1 expression (RNA-seq) in murine leukemic GFI1-36S- and GFI1-36N-MLL-AF9 cells after treatment with 50 μg/mL TMZ for 20 hours and without. Normalized read counts of treated samples were normalized to the untreated samples. n = 3; mean ± SD. (I) NDRG1 protein level was analyzed by immunoblotting in BM cells from GFI1-36S and GFI1-36N leukemic mice without and with TMZ (50 μg/mL) treatment for 24 hours. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001.
MGMT downregulation in GFI1-36N cells due to low levels of NDRG1. (A) Fold change expression of Mgmt in murine GFI1-36S- and GFI1-36N-MLL-AF9 leukemic BM cells at different time points after actinomycin D (10 μg/mL) treatment. Mgmt level was normalized to Hprt and to the untreated controls. n = 3; mean ± SD. (B) NDRG1 protein level in GFI1-36S- and GFI1-36N-MLL-AF9 leukemic BM cells (proteomic). n = 4; mean ± SD. (C) Ndrg1 expression (normalized read counts; RNA-seq) of murine leukemic GFI1-36S- and GFI1-36N-MLL-AF9 BM cells. n = 3; mean ± SD. (D) Ndrg1 gene expression measured in GFI1-36S- and GFI1-36N-MLL-AF9 BM cells by RT-PCR. GFI1-36S: n = 3 and GFI1-36N: n = 3; mean ± SD. (E) Published GFI1-ChIP-seq data sets showing the Ndrg1 gene and its regulatory elements with the possible binding sides of GFI1 (red square) at regulatory elements of Ndrg1. (F) GFI1-ChIP-quantitative PCR of the Ndrg1 upper regulatory elements of murine GFI1-36S and GFI1-36N leukemic BM cells. Gapdh and Runx1 were used as a control (right). (G) Comparison between the GFI1 binding motif from the Jasper database (top) and the consensus motif found using find individual motif occurrence (FIMO) at sites occupied by GFI1 in 21 genes differentially expressed in granulocyte/monocyte progenitors s from GFI1-36N or -36S animals. (H) Ndrg1 expression (RNA-seq) in murine leukemic GFI1-36S- and GFI1-36N-MLL-AF9 cells after treatment with 50 μg/mL TMZ for 20 hours and without. Normalized read counts of treated samples were normalized to the untreated samples. n = 3; mean ± SD. (I) NDRG1 protein level was analyzed by immunoblotting in BM cells from GFI1-36S and GFI1-36N leukemic mice without and with TMZ (50 μg/mL) treatment for 24 hours. ∗P < .05; ∗∗∗P < .001; ∗∗∗∗P < .0001.
NDRG1 (stress-responsive protein involved in DNA damage response) positively regulates and stabilizes MGMT in human glioblastoma cells by direct protein-protein binding.39,40 NDRG1 protein levels were reduced in GFI1-36N leukemic cells (Figure 5B). Additionally, using our RNA-seq data sets from murine leukemic cells, the expression of Ndrg1 was also significantly lower in GFI1-36N leukemic cells than in GFI1-36S leukemic cells (Figure 5C). We validated these results using reverse transcription PCR (Figure 5D). Reanalysis of published GFI1 ChIP-seq data from CODEX (GSE31657, GSE69101, GSE50806, and GSE42518)17,33,37,38 revealed 2 potential GFI1 binding sites at the upper regulatory regions of Ndrg1 in 3 out of 4 data sets (Figure 5E). Although GFI1 has been described as a transcriptional repressor, it can also act as an activator, as demonstrated in the case of medulloblastoma41 or in T-cell leukemia for Ikaros.42,43 Using ChIP–quantitative PCR with a GFI1 targeting antibody, we found that GFI1-36S bound to a higher degree to both upper regulatory elements of Ndrg1 than GFI1-36N in murine leukemic cells (Figure 5E-F). Repeated attempts to examine the binding of GFI1-36S and GFI1-36N on a genome-wide level did not yield sufficient reads; hence, this must be done in future studies. In line with this, PNKP and APEX1, 2 additional proteins bound and stabilized by NDRG1,39 were downregulated at both RNA and protein levels in GFI1-36N compared with in GFI1-36S leukemic cells (supplemental Figure 6G-H). To assess whether decreased binding of GFI1-36N to its target genes was due to a different binding sequence, we analyzed genes differentially expressed between granulocyte/monocyte progenitors from animals carrying either GFI1 variant. The consensus binding sequence of GFI1-36S and GFI1-36N was not different based on selected 21 genes with a GFI1 peak using the program FIMO (Figure 5G)44 and almost identical to the published GFI1-binding consensus motif.45
Next, we treated GFI1-36S and GFI1-36N leukemic cells with temozolomide, leading to the upregulation of Ndrg1 expression in GFI1-36S but not in GFI1-36N leukemic cells (Figure 5H), and this was confirmed at protein levels 24 hours after temozolomide treatment (Figure 5I). These data suggest that GFI1-36S more efficiently binds to regulatory elements in the Ndrg1 locus than GFI1-36N and suggest that GFI1-36S occupation leads to increased Ndrg1 expression. However, more future work is required to fully understand this.
Combination of temozolomide and olaparib is selectively cytotoxic to GFI1-36N leukemic cells in vitro and in vivo
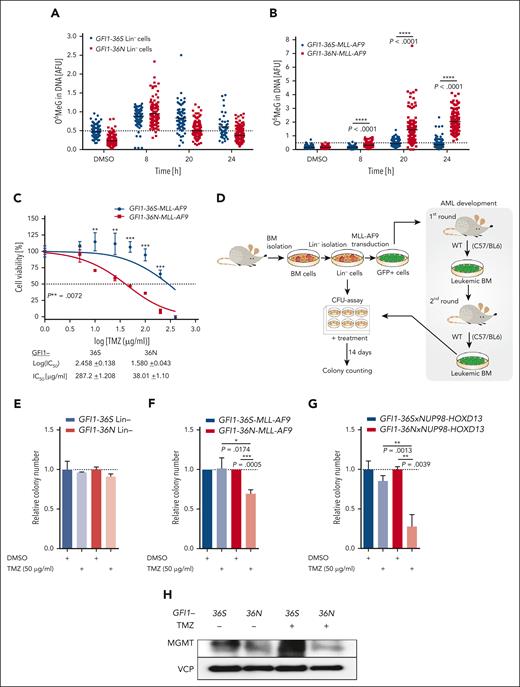
The alkylating agent temozolomide methylates guanine residues at O-6 positions (O6MeG). In a subset of patients with glioblastoma, DNA methylation of the MGMT locus promoter sequences leads to a downregulation of the MGMT protein level, which sensitizes the tumor cells to treatment with temozolomide.32,35,36 A reduced level of MGMT could open the possibility of targeting GFI1-36N–expressing leukemic cells. To probe for this, we explored MGMT-mediated clearance of O6MeG adducts induced by temozolomide but found no difference in nonleukemic Lin– cells of Gfi1-36S and -36N knockin mice (Figure 6A). However, GFI1-36N leukemic cells showed significantly lower efficiency to repair O6MeG than GFI1-36S leukemic cells (Figure 6B). The 50% inhibitory concentration value of temozolomide was eightfold lower in leukemic GFI1-36N cells than in leukemic GFI1-36S cells (Figure 6C). At low concentrations, temozolomide did not affect the expansion of nonleukemic hematopoietic cells (Figure 6D-E; supplemental Figure 7A), but at these same concentrations, temozolomide significantly inhibited the growth of leukemic GFI1-36N cells and caused extensive apoptosis (Figure 6F; supplemental Figure 7B-D). RNA-seq data showed that GFI1-36N leukemic cells were enriched for gene set enrichment analysis terms related to p53 pathway activation and apoptosis after 20 hours of treatment with temozolomide compared with GFI1-36S leukemic cells (supplemental Figure 8). We validated these findings in another murine AML model in which GFI1-36N or GFI1-36S cells were generated that coexpressed the AML oncofusion protein NUP98-HOXD13. Similar to the previously generated MLL-AF9–expressing cells, these NUP98-HOXD13 leukemic cells were more sensitive toward temozolomide when the GFI1-36N allele was present compared with the GFI1-36S allele (Figure 6G; supplemental Figure 9A). Further on, GFI1-36S leukemic cells upregulated MGMT levels after temozolomide treatment, which was not the case for GFI1-36N leukemic cells (Figure 6H).
GFI1-36N leukemic cells are highly susceptible to TMZ treatment. (A) Functional Mgmt assays from murine GFI1-36S and GFI1-36N Lin– cells (45-206 cells per sample) and (B) GFI1-36S and GFI1-36N MLL-AF9 BM cells from mice that received transplantation (115-223 cells per sample). Cells were treated with 100 μg/mL TMZ and at different time points after treatment O6MeG level was analyzed with immunofluorescence. The ACAS program was used for the evaluation. (C) Cell viability of murine GFI1-36S and GFI1-36N MLL-AF9 BM cells measured by MTT assay after treatment with different TMZ concentrations for 48 hours, and IC50 values were calculated; mean ± SD. (D) Schematic experimental setup of the colony-forming unit (CFU) assays. (E) Murine GFI1-36S and GFI1-36N Lin– cells and (F) MLL-AF9 BM cells from mice were plated for 14 days in methylcellulose medium with the addition of 50 μg/mL TMZ or as a control dimethyl sulfoxide (DMSO). The colony number of the treated samples was calculated relative to the control. n = 3; mean ± SD. (G) CFU assay was performed with malignant BM cells from transgenic GFI1-36Sx and GFI1-36NxNUP98-HOXD13 mice. Cells were treated with 50 μg/mL TMZ and as a control with DMSO. The colony number after 14 days in culture of the treated samples was calculated relative to the control. n = 2, each triplicate, mean ± SD. (H) MGMT protein level was measured by immunoblotting in BM of GFI1-36S and GFI1-36N leukemic mice without and with TMZ (50 μg/mL) treatment for 24 hours. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. AFU, arbitrary fluorescence units (O6MeG/4′,6-diamidino-2-phenylindole); IC50, 50% inhibitory concentration.
GFI1-36N leukemic cells are highly susceptible to TMZ treatment. (A) Functional Mgmt assays from murine GFI1-36S and GFI1-36N Lin– cells (45-206 cells per sample) and (B) GFI1-36S and GFI1-36N MLL-AF9 BM cells from mice that received transplantation (115-223 cells per sample). Cells were treated with 100 μg/mL TMZ and at different time points after treatment O6MeG level was analyzed with immunofluorescence. The ACAS program was used for the evaluation. (C) Cell viability of murine GFI1-36S and GFI1-36N MLL-AF9 BM cells measured by MTT assay after treatment with different TMZ concentrations for 48 hours, and IC50 values were calculated; mean ± SD. (D) Schematic experimental setup of the colony-forming unit (CFU) assays. (E) Murine GFI1-36S and GFI1-36N Lin– cells and (F) MLL-AF9 BM cells from mice were plated for 14 days in methylcellulose medium with the addition of 50 μg/mL TMZ or as a control dimethyl sulfoxide (DMSO). The colony number of the treated samples was calculated relative to the control. n = 3; mean ± SD. (G) CFU assay was performed with malignant BM cells from transgenic GFI1-36Sx and GFI1-36NxNUP98-HOXD13 mice. Cells were treated with 50 μg/mL TMZ and as a control with DMSO. The colony number after 14 days in culture of the treated samples was calculated relative to the control. n = 2, each triplicate, mean ± SD. (H) MGMT protein level was measured by immunoblotting in BM of GFI1-36S and GFI1-36N leukemic mice without and with TMZ (50 μg/mL) treatment for 24 hours. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001. AFU, arbitrary fluorescence units (O6MeG/4′,6-diamidino-2-phenylindole); IC50, 50% inhibitory concentration.
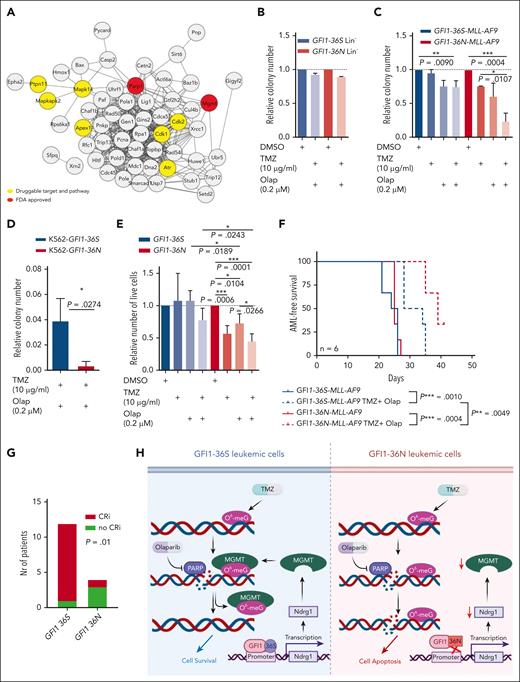
We performed a network analysis of deregulated DNA repair proteins and queried for drugs targeting either the proteins or repair pathways. In this analysis, we found that the use of temozolomide to target MGMT repair pathways or olaparib to target PARP1 would be a possible approach (see details in supplemental Methods for data analysis and bioinformatics; Figure 7A). Olaparib can target cells with HR defects and potentiate the effect of temozolomide.46-49 We observed that a combination of temozolomide and olaparib caused increased apoptosis in GFI1-36S leukemic cells in our system and significantly inhibited the growth of GFI1-36N leukemic cells compared with GFI1-36S leukemic cells without affecting nonleukemic cells in colony assays in vitro (Figure 7B,C; supplemental Figure 9B-E). We also confirmed this effect using K562 cells with a GFI1-36N allele or primary human AML cells from GFI1-36N carriers treated with TMZ and olaparib (Figure 7D-E; supplemental Figure 9F). In both instances, GFI1-36N leukemic cells were highly sensitive to temozolomide, alone or in combination with olaparib. To characterize how the sensitivity to these compounds in GFI1-36N leukemic cells was mediated by downregulated MGMT levels, we assessed AML cell lines for MGMT expression. We performed lentiviral-mediated stable knockdown of MGMT in THP1 cells with the GFI1-36S genotype that showed abundant MGMT protein levels to mimic the phenotype (supplemental Figure 10A-C). Knockdown of MGMT in THP1 cells phenocopied the sensitivity to temozolomide or in combination with olaparib in cell growth and viability assays (supplemental Figure 10D). This is another indication that MGMT levels explain the sensitivity of GFI1-36N leukemic cells to DNA-damaging drugs. Additionally, we tested the sensitivity of GFI1-36N leukemic cells to standard chemotherapeutic agents. We treated another set of primary AML cells from the Dresden cohort (GFI1-36S, n = 15 and GFI1-36N, n = 11) with the drugs. We observed that GFI1-36N cells were more responsive to the drugs daunorubicin, gilteritinib, and venetoclax (supplemental Figure 11A-C), which might be related to the function of GFI1 regulating p53 and associated apoptosis signaling pathways.50,51 However, there was no difference in the treatment response to the drugs cytarabine, azacytidine, sorafenib, and glasdegib (supplemental Figure 11D-G).
The combination of TMZ and olaparib shows synergistic effect on GFI1-36N leukemic cells in vitro and in vivo. (A) Analysis of possible drug targets of the differently expressed DNA repair–related proteins (results from the proteomic analysis of GFI1-36S and GFI1-36N leukemic BM cells). (B) CFU assay results of murine Lin– cells after treatment with either the combination of 10 μg/mL TMZ and 0.2 μM olaparib (Olap) or DMSO as a control. n = 3, mean ± SD (C) CFU assay results from MLL-AF9 BM cells from mice that received transplantation after treatment with either 10 μg/mL TMZ (n = 2), 0.2 μM Olap (n = 2), or the combination of both (n = 3, triplicate) and as a control DMSO (n = 3). Colony number was determined, and treated samples were calculated relative to the control. mean ± SD (D) CFU assay results from K562 cells expressing GFI1-36S and GFI1-36N, treated with 10 μg/mL TMZ and 0.2 μM Olap. Relative colony numbers were calculated with respect to DMSO control (n = 3, triplicate). (E) Primary human GFI1-36S (GFI1-36S/S: 2 × BM and 2 × peripheral blood) and GFI1-36N (GFI1-36S/N: 1 × BM and 1 × SPL and GFI1-36N/N: 2 × peripheral blood) cells from patients with AML were plated 14 days in methylcellulose media and treated as described in (B). Number of live cells was determined, and treated samples were calculated relative to the control. n = 4; mean ± SD. (F) AML-free survival of mice that received transplantatiob with TMZ and Olap treatment or without. GFI1-36S or GFI1-36N MLL-AF9 BM cells were transplanted into sublethally irradiated WT mice and on day 3 after transplantation, the treatment with 100 mg/kg olaparib and 50 mg/kg TMZ was started. n = 6. (G) In an ongoing clinical trial (NCT04207190) of treating patients with AML with talazoparib along with gemtuzumab ozogamicin, 3 out of 4 GFI1-36N patients showed CRi, whereas 1 out of 12 GFI1-36S patients showed CRi. (H) Scheme elucidates GFI1-36N influence on DNA repair and genome stability in AML cells compared with GFI1-36S. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. FDA, Food and Drug Administration; SPL, spleen. Scheme created with BioRender.com.
The combination of TMZ and olaparib shows synergistic effect on GFI1-36N leukemic cells in vitro and in vivo. (A) Analysis of possible drug targets of the differently expressed DNA repair–related proteins (results from the proteomic analysis of GFI1-36S and GFI1-36N leukemic BM cells). (B) CFU assay results of murine Lin– cells after treatment with either the combination of 10 μg/mL TMZ and 0.2 μM olaparib (Olap) or DMSO as a control. n = 3, mean ± SD (C) CFU assay results from MLL-AF9 BM cells from mice that received transplantation after treatment with either 10 μg/mL TMZ (n = 2), 0.2 μM Olap (n = 2), or the combination of both (n = 3, triplicate) and as a control DMSO (n = 3). Colony number was determined, and treated samples were calculated relative to the control. mean ± SD (D) CFU assay results from K562 cells expressing GFI1-36S and GFI1-36N, treated with 10 μg/mL TMZ and 0.2 μM Olap. Relative colony numbers were calculated with respect to DMSO control (n = 3, triplicate). (E) Primary human GFI1-36S (GFI1-36S/S: 2 × BM and 2 × peripheral blood) and GFI1-36N (GFI1-36S/N: 1 × BM and 1 × SPL and GFI1-36N/N: 2 × peripheral blood) cells from patients with AML were plated 14 days in methylcellulose media and treated as described in (B). Number of live cells was determined, and treated samples were calculated relative to the control. n = 4; mean ± SD. (F) AML-free survival of mice that received transplantatiob with TMZ and Olap treatment or without. GFI1-36S or GFI1-36N MLL-AF9 BM cells were transplanted into sublethally irradiated WT mice and on day 3 after transplantation, the treatment with 100 mg/kg olaparib and 50 mg/kg TMZ was started. n = 6. (G) In an ongoing clinical trial (NCT04207190) of treating patients with AML with talazoparib along with gemtuzumab ozogamicin, 3 out of 4 GFI1-36N patients showed CRi, whereas 1 out of 12 GFI1-36S patients showed CRi. (H) Scheme elucidates GFI1-36N influence on DNA repair and genome stability in AML cells compared with GFI1-36S. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. FDA, Food and Drug Administration; SPL, spleen. Scheme created with BioRender.com.
To recapitulate this in vivo, we transplanted GFI1-36S-MLL-AF9 or GFI1-36N-MLL-AF9 leukemic BM cells into sublethal irradiated wild-type mice and treated the mice with a combination of olaparib and temozolomide. The cohorts of treated mice with either genotype survived significantly longer than those that were untreated. However, the treated GFI1-36N-MLL-AF9 mice survived significantly longer (mean, 7 days; P = .0049) than the GFI1-36S-MLL-AF9–treated mice (Figure 7F), again confirming the effect of GFI1-36N in sensitizing AML cells to combination treatment with olaparib and temozolomide. We also searched for clinical trials using temozolomide and/ or a PARP inhibitor. From 5 publicly recorded trials, 1 ongoing trial (NCT04207190) is examining talazoparib given together with gemtuzumab ozogamicin in patients with CD33+ refractory or relapsed AML. In this cohort of patients, a statistically significant different response rate was observed, with only 1 of 12 patients with GFI1-36S homozygous showing complete remission with incomplete recovery (CRi) according to European Leukemia Network recommendation,52 whereas 3 out of 4 patients with GFI1-36N heterozygous or homozygous achieved CRi during the course of treatment (Figure 7G).
Discussion
Alterations of DNA repair pathways contribute to the development of various solid cancer entities as well as hematologic malignancies.53-56 GFI1 regulates DNA repair by coordinating the methylation of MRE11 and 53BP1. Consecutively, the loss of GFI1 affects HR but not NHEJ.18 In line with this, GFI1-36N leukemic cells are compromised with regard to HR and MGMT but not for NHEJ-mediated repair. Molecularly, GFI1 transcriptionally regulates the activity of MGMT by activating the expression of NDRG1, a protein that stabilizes MGMT.39 Although GFI1 has been originally described as a transcriptional repressor, it also associates with coactivators and other transcription factors, such as Ikaros, or with components of the nucleosome remodeling deacetylase complex to upregulate the expression of respective target genes.42,43,57 GFI1-36N fails to bind to the same extent to regulatory elements of Ndrg1 as GFI1-36S. This could explain the lower levels of MGMT in cells expressing the GFI1-36N variant and the failed upregulation of Ndrg1 after treatment of GFI1-36N–expressing cells with temozolomide.
Both in human and murine cells, the presence of GFI1-36N is associated with a mutational signature because of an altered DNA-repair capacity. We also observed a higher susceptibility of GFI1-36N cells to DNA damage than of GFI1-36S cells. This might be partly due to more open chromatin as a result of increased H3K9 acetylation and H3K4 dimethylation, as reported before for GFI1-36N cells.9,17 This explanation is consistent with other studies that have reported a correlation between the number of open chromatin states and susceptibility to DNA damage.58 In summary, the presence of GFI1-36N in leukemic cells leads to higher chromatin accessibility and reduced HR- and MGMT-mediated DNA repair.
Targeting MGMT via temozolomide with tumors coopting mutations in the DNA damage repair gene was shown to achieve an exceptional response to cancer therapy.59 Both HR-directed and MGMT-mediated DNA repair pathways have been targeted in different cancer entities using temozolomide and/or olaparib.32,47,49,60-62 Temozolomide alone or in combination with olaparib significantly reduced the expansion of murine and human GFI1-36N leukemic cells in vitro and in vivo without affecting nonleukemic cells. Finally, use of another PARP inhibitor induced in an albeit small cohort a 75% rate of CRi among patients with heterozygous or homozygous GFI1-36N. PARP inhibitors and temozolomide in combination with, for example, cytotoxic therapy have well-manageable side effects, which could prove to be particularly beneficial for older patients with GFI1-36N–positive AML. Although temozolomide and olaparib can induce further mutations, which is also true for other chemotherapeutic approaches, PARP inhibitors and alkylating agents offer the possibility of selectively targeting GFI1-36N leukemic cells in potential future trials.
Acknowledgments
The authors thank the Core Facility Genomics of the University Muenster and Genomics and Transcriptomics Facility for RNA sequencing. Furthermore, the authors also thank the Imaging Center Essen and the Fluorescence Microscopy Facility Münster for guidance and providing the microscopes. The authors also thank Maria Eynck, Renata Köster, Dagmar Clemens, Hannelore Leuschke, and Claudia Dill for their technical assistance, and Klaus Lennartz and Thorsten König for their assistance with cell sorting. The authors thank the members of Proteomics and Signal Transduction Department at the Max Planck Institute for Biochemistry, particularly Igor Paron for his technical support. H.B. and A.K. acknowledge computational support from the OMICS compute cluster at the University of Lübeck.
The work was supported by the Deutsche Krebshilfe (70112392) and partially by the Jose Carreras Leukämie Stiftung (DJCLS 17R/2018), Deutsche Forschungsgemeinschaft (KH331/2-3), and the intramural funding of the faculty of Medicine at University Hospital of Muenster (Kha2/002/20). This study was supported by AstraZeneca and Merck Sharp & Dohme Corp, a subsidiary of Merck & Co Inc, Kenilworth, NJ, who are codeveloping olaparib. J. Vorwerk was supported by the Jürgen Manchot Foundation and the Medizinerkolleg Münster. A.K.J. and M.M. were supported by the Max Planck Society for the Advancement of Science and by the German Research Foundation (Gottfried Wilhelm Leibniz Prize). A.K.J. is supported by DFG Emmy Noether grant (JA3274/1-1). H.B. was supported by the Deutsche Forschungsgemeinschaft (German Research Foundation) under Germany's Excellence Strategy EXC 22167-390884018, partially funded by the Bundesministerium für Bildung und Forschung grant 01ZZ1804B (DIFUTURE) (A.M.N.B.).
Authorship
Contribution: D.F., J. Vorwerk, L.M., L.M.G., N.J., Y.A.-M., P.K.P., H.M.M.A., L.L., X.X., K.S., L.M.G., E.W., J.T., F.S., L.R., and A.K.J. performed experimental research; D.F., F.C.C., H.A., M.G., M.S., and D.K. genotyped human patient samples; D.F., J. Vorwerk, J.H., and F.N. took the fluorescence images; D.F., J.M.F., A.K., H.M., S.H., L.W., V.C., E.W., S.K.M., J.W.T., A.K.J., T.L., and C.K. performed data analysis, presentation, and interpretation; A.F. performed data analysis and edited the manuscript; C.R. provided samples, performed data analysis, and edited the manuscript; N.v.B. and G.L. provided funding and samples, analysed data, and edited the manuscript; A.M.N.B., R.A., and T.H. performed bioinformatics analyses; H.B., G.H., M.D., M.K., and J. Varghese supervised and supported bioinformatics analyses; R.R. provided essential mouse strains and performed the analysis of the mouse model; J.T. performed the immunostaining, the measurements and the evaluation of the O6MeG assays; A.K.J., L.M., M.K., T.H., and J.M.F. performed mass spectrometry experiments, data analysis, and bioinformatics; A.K.J. and M.M. provided support and supervised the mass spectrometry experiments; V.C. and T.M. analyzed GFI1-binding sites in hematopoietic precursor cells; M.H., F.T., G.G., D.S., J.T., F.S., W.E.B., J.K., F.R., A.K., M.D., U.D., M.M., A.K.J., M.H., J. Vorwerk, H.C.R., and C.K. provided essential data or samples or research support; D.F., A.K.J., and C.K. designed the study and wrote the manuscript; S.C.N., B.O., and T.M. critically revised the article; and all authors read, provided critical comments, and approved the manuscript.
Conflict-of-interest disclosure: H.C.R. received consulting and lecture fees from AbbVie, AstraZeneca, Vertex, and Merck; received research funding from Gilead Pharmaceuticals; and is a cofounder of CDL Therapeutics GmbH. G.H. received consulting and lecture fees from Novartis, Incyte, and Jazz Pharmaceuticals. C.K. received funding from AstraZeneca for this project. E.W. received funding from Pfizer for conducting the clinical trial. The remaining authors declare no competing financial interests.
B.O. and U.D. are retired from University Hospital Essen, Essen, Germany.
Correspondence: Matthias Mann, Department of Proteomics and Signal Transduction, Max Planck Institute of Biochemistry, Am Klopferspitz 18, 82152 Martinsried, Germany; email: mmann@biochem.mpg.de; Ashok Kumar Jayavelu, Proteomics and Cancer Cell Signaling, Deutsches Krebsforschungszentrum, Im Neuenheimer Feld 280, 69120 Heidelberg, Germany; email: ak.jayavelu@dkfz-heidelberg.de; and Cyrus Khandanpour, Department of Hematology and Oncology, University Cancer Center Schleswig- Holstein , University Hospital of Schleswig-Holstein Campus Lübeck and University of Lübeck, Ratzeburger-Allee 160, 23564 Luebeck, Germany; email: cyrus.khandanpour@uksh.de.
References
Author notes
RNA-seq data are deposited in the Gene Expression Omnibus database (accession number GSE195955). Proteome data are deposited in the proteome repository (accession number PXD037433).
Other original data are available on request from the corresponding authors, Matthias Mann (mmann@biochem.mpg.de), Ashok Kumar Jayavelu (ak.jayavelu@dkfz-heidelberg.de), and and Cyrus Khandanpour (cyrus.khandanpour@uksh.de).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![More genetic aberrations in human and murine GFI1-36N AML samples. (A) Percentage of patients with MDS/AML of 3 different cohorts with >2 chromosomal aberrations. Patient samples were genotyped for the presence of GFI1-36N or GFI1-36S with real time (RT)-PCR. (B) Number of patients in individual cohorts corelating to gender and age of the patients. (C) Schematic experimental setup to generate leukemic mice and the serial transplantation experiments. (D) Serial transplanted BM cells from leukemic MLL-AF9 mice and nonleukemic Lin– cells were analyzed using RNA-seq followed by variant calling analysis. Shown is the number of variations in leukemic cells minus the number of variations in nonleukemic cells. n = 3; mean ± standard deviation. (E) Variations from (D) divided according to the functional class of mutation. Shown is the total number of mutations per genotype (left). The Venn diagram (right) represents the overlaps of missense mutations between GFI1-36S and GFI1-36N leukemic cells. (F) Scheme of the PiggyBac transposon-based mouse model. GFI1-36S or GFI1-36N mice were crossed with the PiggyBac transposon mice (Mx-Cre × Rosa26 × ATP2). Mice were injected with poly(I:C) to activate the transposon system. (G) The PiggyBac transposon-based mouse model was used to check the number of common insertion sites (CISs) of the transposon sequence. The number of CISs were calculated for each genotype. WT: n = 4, GFI1-36S (heterozygous [n = 6] and homozygous [n = 1]): n = 7, and GFI1-36N (heterozygous [n = 2] and homozygous [n = 7]): n = 9. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001. M, men; W, women; WT, wild-type.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/142/25/10.1182_blood.2022015752/2/m_blood_bld-2022-015752-gr1.jpeg?Expires=1770802190&Signature=bH2pZCBxgz~YQ4spKZZMlZi70ZMYicLRNeNU5vKMw~sYyXGlAM3-9gobo5y3tFLHgD0W~R1D-qlW9RI1oPksKoc8nkwxLFJjIbRp2uIqUd6gqmheCxQki7PIiRSZAz7J5lnypw3IFQ55JZQ487Ji5zeM7EIjUbAOvMsRn56xs5gfaxlbA9fKi53fagM9tWA7DrlzEsTq34POnqXVz4GzrVKMqqf8xgVwrCedyhq-Bt9RQohcEPvIA4TjktclJH1qx86Iwt7vurpCNe~X6hDtg7Vl1qN~I5vVUElpkfp1aH1XcfV2l4sMfaHdF0coF9FqeSOPLU3jWXkTeuN11oRygw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal