Issue Archive
Table of Contents
BLOOD COMMENTARIES
PLENARY PAPER
CEBPA repression by MECOM blocks differentiation to drive aggressive leukemias
High-risk acute myeloid leukemia (AML) frequently exhibits increased expression of the transcription factor MDS1 and EVI1 complex locus protein (MECOM), which is essential for hematopoietic stem cells in healthy states. Two independent studies in this issue, a Plenary Paper by Fleming et al and a Brief Report by Pastoors et al, describe a simple mechanism whereby the MECOM protein preserves the leukemic state by binding the lineage-restricted +42 kb enhancer of the CCAAT enhancer-binding protein α (CEBPA) gene. Importantly, removing that repression through MECOM degradation rapidly restores CEBPA expression, triggering terminal myeloid differentiation. These insights lay the groundwork for potential new differentiation therapies for high-risk AML.
CLINICAL TRIALS AND OBSERVATIONS
LEF1 intragenic deletion induces a dominant-negative isoform and unveils a Wnt/β-catenin vulnerability in T-ALL
Clinical Trials & Observations
Inactivation of lymphoid enhancer-binding factor 1 (LEF1) has been associated with T-acute lymphoblastic leukemia (T-ALL), particularly in young patients, but the mechanism of oncogenesis remains unknown. In this study, Delafoy and colleagues report that focal intragenic deletions of LEF1 are common in T-ALL and generate a previously undescribed dominant-negative Δ2/3 isoform that disrupts Wnt/β-catenin signaling, impairs glucocorticoid receptor responsiveness, and confers enhanced sensitivity to combined cyclosporin A and glucocorticoid therapy. These important findings identify LEF1 deletions not only as a mechanistic driver of disease but also as a potentially actionable biomarker of therapeutic vulnerability in T-ALL.
Prognostic value of premaintenance FDG PET/CT response in patients with newly diagnosed myeloma from the CASSIOPEIA trial
CME
Clinical Trials & Observations
Quadruplet therapy before maintenance has become the standard of care for many patients with newly diagnosed multiple myeloma (MM), but complete responses are not universal. In this month’s CME paper, Kraeber-Bodéré and colleagues report the results of CASSIOPET, the imaging companion study of the CASSIOPEIA trial, showing that achieving negativity in positron emission tomography (PET) before initiation of maintenance therapy remains an important prognostic marker for patients with MM, even those who achieved minimal residual disease negativity in the bone marrow.
More than an emotional support PET
Clinical Trials & Observations
IMMUNOBIOLOGY AND IMMUNOTHERAPY
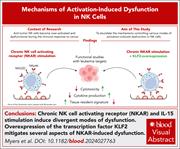
Chronic NK cell activation results in a dysfunctional, tissue resident–like state mediated by KLF2 deficiency
Immune exhaustion can be a major limiting factor for cancer immunotherapy. Myers and colleagues report a study on mouse and human natural killer (NK) cells which shows that chronic activation of NK cells, via the NK cell activation receptor, results in compromised function and an altered NK cell state that affects tissue homing. Mechanistically, they demonstrate that modulating expression of the transcription factor Krüppel-like factor 2 (KLF2) prevents some of the features of chronic activation.
LYMPHOID NEOPLASIA
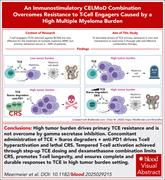
An immunostimulatory CELMoD combination overcomes resistance to T-cell engagers caused by a high multiple myeloma burden
Bispecific T-cell engagers (TCEs) targeting B-cell maturation antigen (BCMA) and CD3 induce responses in heavily pretreated patients with multiple myeloma (MM), but high tumor burden leads to resistance. Using an immunocompetent murine model of MM, Meermeier and colleagues demonstrate that pretreatment with cereblon E3 ligase modulatory drugs (CELMoDs) expands the bone marrow T-cell compartment and overcomes primary resistance to anti-BCMA TCEs by outnumbering MM cells. Ongoing clinical trials using this strategy should determine the therapeutic relevance of this approach.
Genomic landscape of IgM-MGUS and patients with stable or progressive asymptomatic Waldenström macroglobulinemia
Determining the risks of progression of immunoglobulin M monoclonal gammopathy of undetermined significance (IgM-MGUS) and asymptomatic Waldenström macroglobulinemia (WM) to symptomatic WM remains a challenge. Bagratuni and colleagues developed a multistep clinical model of WM coupled with whole-exome sequencing (WES) to aid prognostication. Applying machine learning to the WES data, they generated and validated a risk score that leads to a newly proposed multistep model of disease progression. Prospective studies should determine the clinical utility of this model.
MYELOID NEOPLASIA
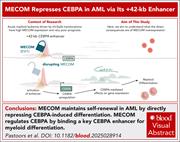
MECOM is a master repressor of myeloid differentiation through dose control of CEBPA in acute myeloid leukemia
Brief Report
High-risk acute myeloid leukemia (AML) frequently exhibits increased expression of the transcription factor MDS1 and EVI1 complex locus protein (MECOM), which is essential for hematopoietic stem cells in healthy states. Two independent studies in this issue, a Plenary Paper by Fleming et al and a Brief Report by Pastoors et al, describe a simple mechanism whereby the MECOM protein preserves the leukemic state by binding the lineage-restricted +42 kb enhancer of the CCAAT enhancer-binding protein α (CEBPA) gene. Importantly, removing that repression through MECOM degradation rapidly restores CEBPA expression, triggering terminal myeloid differentiation. These insights lay the groundwork for potential new differentiation therapies for high-risk AML.
RED CELLS, IRON, AND ERYTHROPOIESIS
Rbm38 deficiency impairs erythroid heme biosynthesis and induces porphyria via reduced ferrochelatase expression
The role of the RNA-binding protein Rbm38 in terminal erythropoiesis in vivo is not well understood. By developing and characterizing novel mouse models of constitutive and hematopoietic-specific Rbm38 deficiency, Xie and colleagues elucidated that Rbm38 is a lineage-specific posttranscriptional regulator of the porphyrin enzyme ferrochelatase and erythroid heme biosynthesis. Furthermore, they show that common human genetic variants in RBM38 affect erythrocyte protoporphyrin levels, suggesting a role for these variants as genetic modifiers of unexplained porphyrias.
LETTER TO BLOOD
BLOOD WORK
CONTINUING MEDICAL EDUCATION (CME) QUESTIONS
-
Cover Image
Cover Image
![issue cover]()
Immunofluorescence staining of active β-catenin (red) in the T-cell acute lymphoblastic leukemia (T-ALL) cell line, CCRF-CEM. Lymphoid enhancer factor intragenic deletions produce a dominant-negative isoform and unveil a Wnt/β-catenin vulnerability in T-ALL. See the article by Delafoy et al on page 3036.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals








Breaking the silence: restoring CEBPA to fight leukemia