Issue Archive
Table of Contents
BLOOD COMMENTARIES
REVIEW ARTICLE
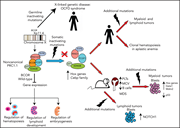
BCOR gene alterations in hematologic diseases
Sportoletti et al provide an authoritative review of how alterations in the BCL6 corepressor (BCOR) gene are involved in the pathogenesis of malignant and nonmalignant hematologic diseases. BCOR and its homologue BCORL1 are transcription factors that act as tumor suppressors. The review summarizes emerging evidence about how alterations in these genes influence the pathophysiology of myeloid and lymphoid neoplasms, as well as aplastic anemia and erythrocytosis.
CLINICAL TRIALS AND OBSERVATIONS
Characterization of HLH-like manifestations as a CRS variant in patients receiving CD22 CAR T cells
Clinical Trials & Observations
Lichtenstein and colleagues characterized an increasingly recognized variant of cytokine release syndrome (CRS) following CD22-targeting chimeric antigen receptor (CAR) T-cell therapy. In a study of a series of 59 patients, they report approximately that one-third of the patients experienced difficult-to-treat hemophagocytic lymphohistiocytosis (HLH)–like manifestations, especially in those with preexisting natural killer cell lymphopenia and higher CAR T-cell expansion and persistence. This research provides clues to identifying those at most risk and suggests roles for anticytokine therapies beyond those directed against interleukin-6.
HEMATOPOIESIS AND STEM CELLS
IMMUNOBIOLOGY AND IMMUNOTHERAPY
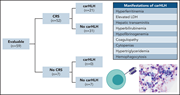
CAR-HEMATOTOX: a model for CAR T-cell–related hematologic toxicity in relapsed/refractory large B-cell lymphoma
The hematologic toxicity of chimeric antigen receptor (CAR) T-cell therapy is widely recognized, but which patients are at highest risk for major prolonged hematological toxicity is an open question. Rejeski et al report that baseline cytopenias and presence of an inflammatory state are associated with prolonged neutropenia. They report a readily applicable risk stratification tool to assist identification of those at low and high likelihood of this complication.
LYMPHOID NEOPLASIA
The protein landscape of chronic lymphocytic leukemia
Meier-Abt and colleagues provide a high-resolution map of the protein landscape of chronic lymphocytic leukemia (CLL) that integrates genetics, RNA expression, methylation, and drug response data. Their work indicates that the protein landscape of CLL is largely governed by 2 genetic factors, trisomy 12 and immunoglobulin heavy-chain variable region mutational status, that in turn unexpectedly implicate upregulated STAT2 activity in chemokine and cytokine signaling responses.
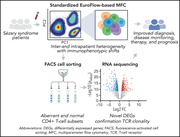
Improved Sézary cell detection and novel insights into immunophenotypic and molecular heterogeneity in Sézary syndrome
PHAGOCYTES, GRANULOCYTES, AND MYELOPOIESIS
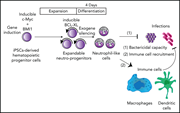
Efficient production of human neutrophils from iPSCs that prevent murine lethal infection with immune cell recruitment
Manufacturing sufficient neutrophils for clinical benefit in patients with chronic severe neutrophil deficiency is a long-sought goal in cellular therapy. Miyauchi et al report the clinically applicable scale production of neutrophil-like cells from genetically engineered human neutrophil–primed progenitors derived from induced pluripotent stem cells (iPSCs). In multiple murine models, these cells display typical neutrophil functions and recruit native immune cells to prevent fulminant infection.
TRANSFUSION MEDICINE
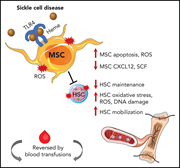
Murine bone marrow mesenchymal stromal cells have reduced hematopoietic maintenance ability in sickle cell disease
The bone marrow microenvironment in sickle cell disease (SCD) is known to be unfavorable to hematopoietic stem cell (HSC) function. Using a transgenic murine model of SCD, Tang et al identified defects in bone marrow mesenchymal stromal cells (MSCs) and HSCs related to chronic heme exposure. Some MSC dysfunction appears reversible through transfusion or via treatment with Nacetylcysteine or TLR4 inhibition. These data may inform optimization of future therapy for SCD.
TRANSPLANTATION
Increased efficacy of dual proinflammatory cytokine blockade on acute GVHD while maintaining GVT effects
Brief Report
Proinflammatory cytokines, including interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α), are important contributors to graftversus-host disease (GVHD) pathogenesis. However, randomized clinical trials of blockade of single cytokines as prophylaxis or therapy have been disappointing. In work that suggests potential next clinical steps, Khuat and colleagues used murine models to demonstrate that the dual blockade of TNF-α and IL-6 receptor provides a significant advantage over single-cytokine blockade in protection from severe GVHD yet does not impair graft-versus-tumor (GVT) effects.
LETTER TO BLOOD
BLOOD WORK
-
Cover Image
Cover Image
![issue cover]()
Neutrophil extracellular trap formation of human neutrophils and neutrophil-like cells (NeuCs) from human induced pluripotent stem cells after phorbol myristate acetate stimulation. Engineered progenitors produce NeuCs, on a clinically applicable scale, that can prevent lethal infections in murine models via immune cell recruitment. See the article by Miyauchi et al on page 2555.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Back MatterBack Matter
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals







CAR T-cell hematotoxicity: is inflammation the key?