Issue Archive
Table of Contents
EDITORIAL
Introduction to a How I Treat series on transfusion medicine
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
BLOOD COMMENTARIES
HOW I TREAT SERIES
How I manage major hemorrhage
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
How I treat challenging transfusion cases in sickle cell disease
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
How I use noninvasive prenatal testing for red blood cell and platelet antigens
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
How I manage pregnant patients who are alloimmunized to RBC antigens
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
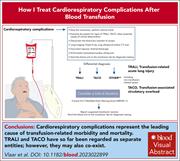
How I diagnose and treat cardiorespiratory complications of transfusion
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
How I treat patients who are refractory to platelet transfusions
Transfusions are part of standard care in many areas across medicine and are the “bread and butter” of practice for many hematologists, yet high-quality evidence is lacking in many areas. Consequently, expert opinion that sifts through lower-quality evidence to provide guidance for those who only are infrequently faced with the following scenarios is invaluable. Associate Editor Erica M. Wood introduces this 6-piece How I Treat series commissioned from leaders in the field. Callum and colleagues illustrate how coordination between diagnosis and therapy is essential when dealing with major hemorrhage and that a multidisciplinary team approach is most effective. Patients with sickle cell disease may receive large numbers of blood products, and challenges are common. Chou and Hendrickson take us through their approach to management and prevention of the real-world problems posed in this group of patients. van ’t Oever et al discuss how to optimally use prenatal testing for cell-free fetal DNA in maternal plasma to reduce the impact of alloimmunization in pregnancy, and Savoia and coauthors further explore the issues that arise in the management of women and babies where pregnancies are affected by red cell alloimmunization. Distinguishing between the various causes of cardiorespiratory distress after transfusion can be very difficult, so Vlaar et al provide a practical approach to address this situation. Finally, we all know that refractoriness to platelet transfusion is common, but how to identify and deploy the most appropriate and effective solution is not widely understood. To assist, Nahirniak and colleagues address their approach to treat a range of immune and nonimmune causes.
CLINICAL TRIALS AND OBSERVATIONS
Three-year follow-up analysis of first-line axicabtagene ciloleucel for high-risk large B-cell lymphoma: the ZUMA-12 study
Clinical Trials & Observations
Chavez et al present the long-term results of the phase 2, single-arm ZUMA-12 study, evaluating axicabtagene ciloleucel (axi-cel) as part of first-line therapy in patients with high-risk large B-cell lymphoma (LBCL). After a median follow-up of almost 4 years, the complete response rate was 86%, and the 3-year progression-free survival was estimated to be 75%. With no relapses observed 18 months postinfusion, these promising results raise the importance for the field of the ongoing phase 3 study addressing the question of whether first-line axi-cel is superior to standard chemoimmunotherapy for high-risk LBCL.
Low rates of chronic graft-versus-host disease with ruxolitinib maintenance following allogeneic HCT
Clinical Trials & Observations
Brief Report
Preventing graft-versus-host disease (GVHD) while preserving graft-versus-leukemia activity is a holy grail of allogeneic hematopoietic cell transplantation (HCT). Ruxolitinib is a proven therapy for both acute and chronic GVHD, prompting its evaluation as prophylaxis with tacrolimus and methotrexate in this phase 2 multicenter trial for patients undergoing reduced intensity HCT. DeFilipp and colleagues report on a 70% 1-year rate of GVHD-free, relapse-free survival and low incidences of severe acute and chronic GVHD, thereby providing a strong rationale for randomized phase 3 studies of ruxolitinib as part of GVHD prophylaxis.
Prevention is better than cure
Clinical Trials & Observations
HEMATOPOIESIS AND STEM CELLS
A new severe congenital neutropenia syndrome associated with autosomal recessive COPZ1 mutations
The clinical phenotype known as severe congenital neutropenia is a manifestation of genetic disorders in many different pathways governing granulopoiesis. A new cause is revealed by Borbaran Bravo and colleagues, who describe 2 unrelated families with symptomatic severe neutropenia with homozygous truncating or missense mutations in coatomer protein complex I subunit zeta 1 (COPZ1). The resultant abnormal trafficking between the Golgi apparatus and endoplasmic reticulum leads to aberrant cell signaling and gene expression, causing myelopoiesis failure.
LYMPHOID NEOPLASIA
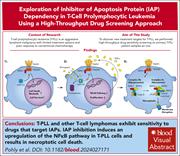
IAP dependency of T-cell prolymphocytic leukemia identified by high-throughput drug screening
Sustained bone marrow and imaging MRD negativity for 3 years drives discontinuation of maintenance post-ASCT in myeloma
CME
Clinical Trials & Observations
Most patients with newly diagnosed multiple myeloma treated with modern induction regimens, autologous transplantation, and lenalidomide maintenance have long-term progression-free survival, but indefinite maintenance therapy is burdensome. In this month’s CME article, Terpos et al report on a prospective study of cessation of maintenance in patients with sustained minimal residual disease negativity and positron emission tomography negativity after 3 years of maintenance. After a median of 3 years postcessation, 75.8% of these patients remained treatment-free, and 93% remained alive and without clinical progression, suggesting that a substantial minority of patients can safely cease maintenance.
MYELOID NEOPLASIA
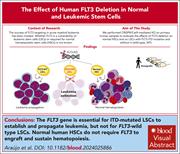
FLT3 is genetically essential for ITD-mutated leukemic stem cells but dispensable for human hematopoietic stem cells
PLATELETS AND THROMBOPOIESIS
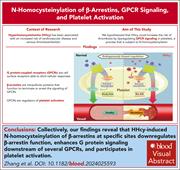
N-homocysteinylation of β-arrestins biases GPCR signaling and promotes platelet activation
THROMBOSIS AND HEMOSTASIS
Therapeutic switch from plasma to recombinant ADAMTS13 for patients with congenital TTP from Japanese real-world data
Brief Report
Congenital thrombotic thrombocytopenic purpura (TTP) is ultrarare, due to mutations in ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), and its acute episodes can be treated with infusions of fresh-frozen plasma (FFP). Recombinant ADAMTS13 is replacing prophylactic FFP for patients with frequently recurrent episodes, but the registration trials excluded patients with severe end-organ damage. Sakai and colleagues report the successful transition of 14 patients, 5 with end-stage renal failure, from biweekly FFP to recombinant ADAMTS13, documenting higher levels of ADAMTS13 1 week after treatment and better acceptability.
BLOOD WORK
CONTINUING MEDICAL EDUCATION (CME) QUESTIONS
-
Cover Image
Cover Image
![issue cover]()
A Wright-Giemsa–stained bone marrow smear from a patient with a novel form of severe congenital neutropenia associated with an autosomal recessive truncating mutation in the coatamer protein complex I subunit zeta 1 gene. See the article by Borbaran Bravo et al on page 2317.
- PDF Icon Front MatterFront Matter
- PDF Icon Table of ContentsTable of Contents
- PDF Icon Editorial BoardEditorial Board
Advertisement intended for health care professionals
Email alerts
Advertisement intended for health care professionals













CARs in pole position: ready for the race?
Clinical Trials & Observations